Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Oct 21, 2014; 20(39): 14105-14125
Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14105
Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14105
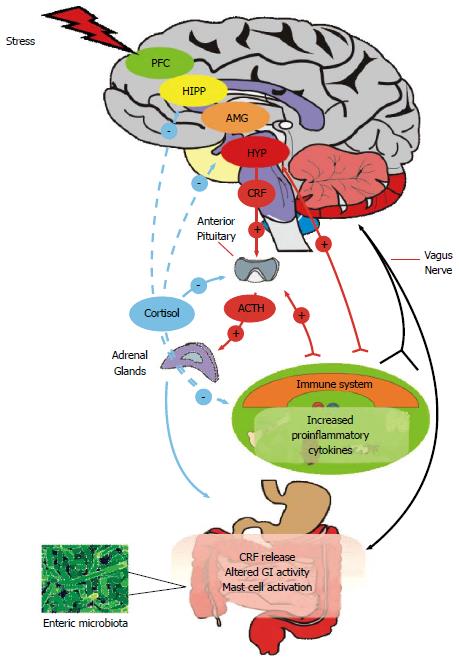
Figure 1 Microbiome-gut-brain Axis.
The central nervous system (CNS) and enteric nervous system (ENS) communicate along vagal and autonomic pathways to modulate many gastrointestinal (GI) functions. The enteric microbiota influence the development and function of the ENS and immune system which affects CNS function. The hypothalamic pituitary adrenal (HPA) axis forms a key component of brain-gut signalling, responding to stress or heightened immune activity. Mood and various cognitive processes can mediate top-down bottom / bottom-up signalling. The HPA axis can be activated in response to environmental stress or by elevated systemic proinflammatory cytokines. Cortisol released from the adrenal glands feeds back to the pituitary, hypothalamus (HYP), amygdala (AMG), hippocampus (HIPP) and prefrontal cortex (PFC) to shut off the HPA axis. Cortisol released from the adrenals has a predominantly anti-inflammatory role on the systemic and GI immune system. In response to stress, GI activity can be altered and corticotropin releasing factor (CRF) increased. Stress can increase systemic proinflammatory cytokines which can act at the pituitary to activate the HPA axis and can signal to the central nervous system via the vagus nerve, which also transmits changes due to mast cell activation in the GI tract.
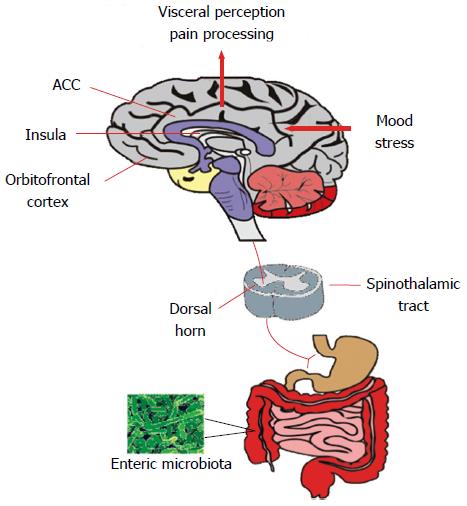
Figure 2 Visceral pain perception.
The microbiota can influence the spinothalamic projections from the gastrointestinal tract which reach higher cortical areas including the insula, anterior cingulate cortex and orbitofrontal cortex where visceral sensory and pain signals reach the conscious awareness. These regions mediate the cognitive processing of visceral signals and integrate mood and stress-related information and initiate autonomic and behavioural responses. ACC: Anterior cingulate cortex.
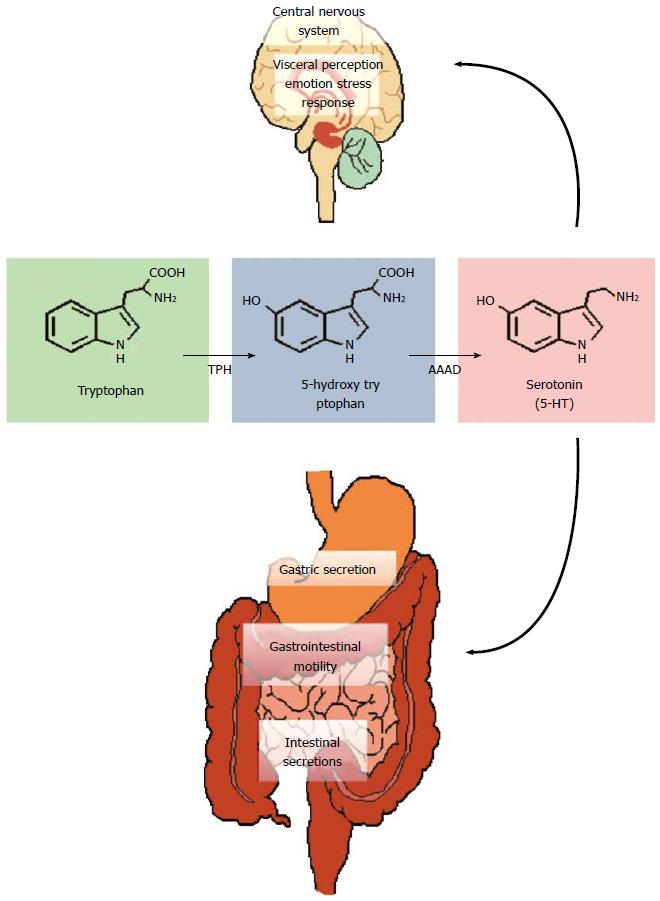
Figure 3 Tryptophan metabolism.
Tryptophan is converted to 5-hydroxytryptophan by tryptophan hydroxylase (TPH) and this is the rate limiting step in the pathway. Aromatic amino acid decarboxylase (AAAD) subsequently converts 5-HTP into serotonin (5-HT). These reactions occur both in the central nervous system (CNS) (where 5-HT regulates a myriad of functions including emotion, cognition, stress and visceral perception) and in the enteric nervous system (gastrointestinal motility and secretion).
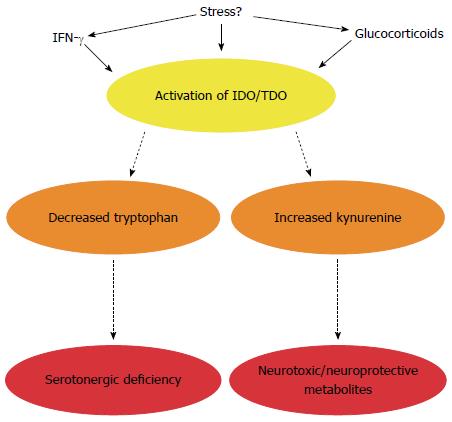
Figure 4 Impact of altered tryptophan metabolism in irritable bowel syndrome.
In addition to serotonin, tryptophan can also be metabolised along the kynurenine pathway to generate neurotoxic and neuroprotective metabolites. The enzymes responsible for degradation along this pathway are immune (indoleamine-2,3-dioxygenase, IDO) and stress (tryptophan-2,3-dioxygenase, TDO) responsive. In IBS, this pathway is activated leading to a potential serotonergic deficiency and/or altered enteric nervous system (ENS) and central nervous system (CNS) availability of kynurenine and its metabolites. The microbiota appears to directly or indirectly regulate enzyme activity.
- Citation: Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J Gastroenterol 2014; 20(39): 14105-14125
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14105.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14105