Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. May 21, 2014; 20(19): 5746-5759
Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5746
Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5746
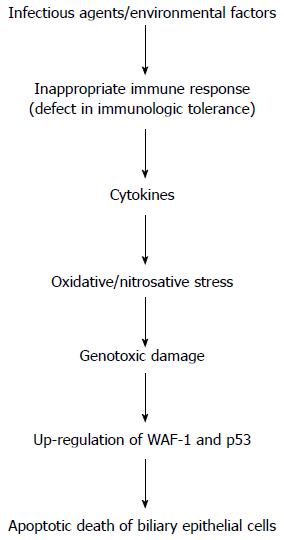
Figure 1 Flow-chart illustrating the cascade of events playing a potential role in the pathogenesis of liver injury in primary biliary cirrhosis.
Important steps include the altered immune response linked to increased oxidative/nitrosative stress and genotoxic damage. The final result is the apoptotic death of biliary epithelial cells. WAF1: Cyclin-dependent kinase inhibitor 1; p53: Tumor protein 53.
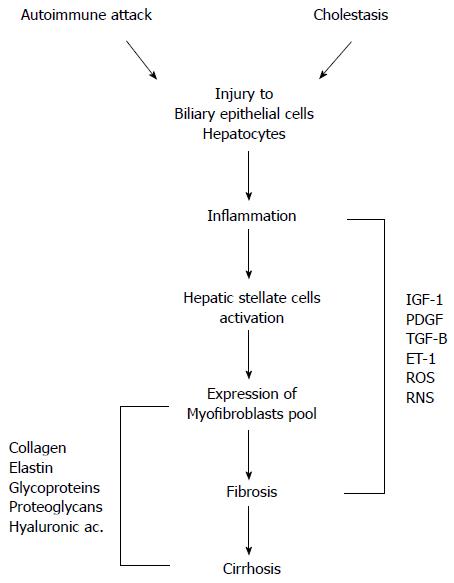
Figure 2 Pathophysiological mechanisms of liver damage in primary biliary cirrhosis.
Autoimmune attack and cholestasis are both responsible for the injury to biliary epithelial cells and hepatocytes. Cell damage results in inflammatory processes with activation of hepatic stellate cells and expression of myofibroblasts leading to fibrosis and cirrhosis. A myriad of mediators have a role in these events. IGF-1: Insulin-like growth factor-1; PDGF: Platelet-derived growth factor; TGF-β: Transforming growth factor beta; ET-1: Endothelin-1; ROS: Reactive oxygen species; RNS: Reactive nitrogen species.
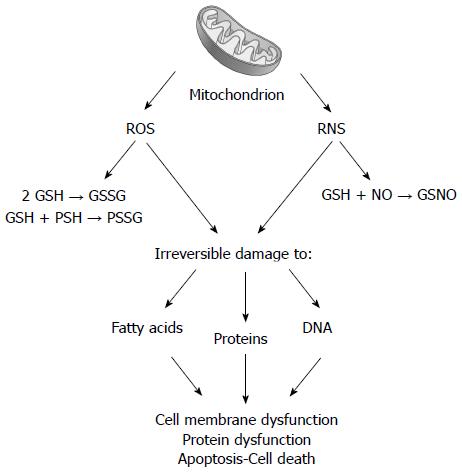
Figure 3 Following chronic cholestasis, liver mitochondrial impairment is associated with increased delivery of reactive oxygen species and reactive nitrogen species.
Reactive oxygen species (ROS) promotes the oxidation of glutathione (GSH to GSSG) and of protein sulfhydryls (PSH to PSSG). Reactive nitrogen species (RNS) favors the formation of nitrosothiols (GSNO). Both steps are responsible for irreversible damage to lipids, proteins, and nucleic acids, ultimately leading to membrane and protein alteration.
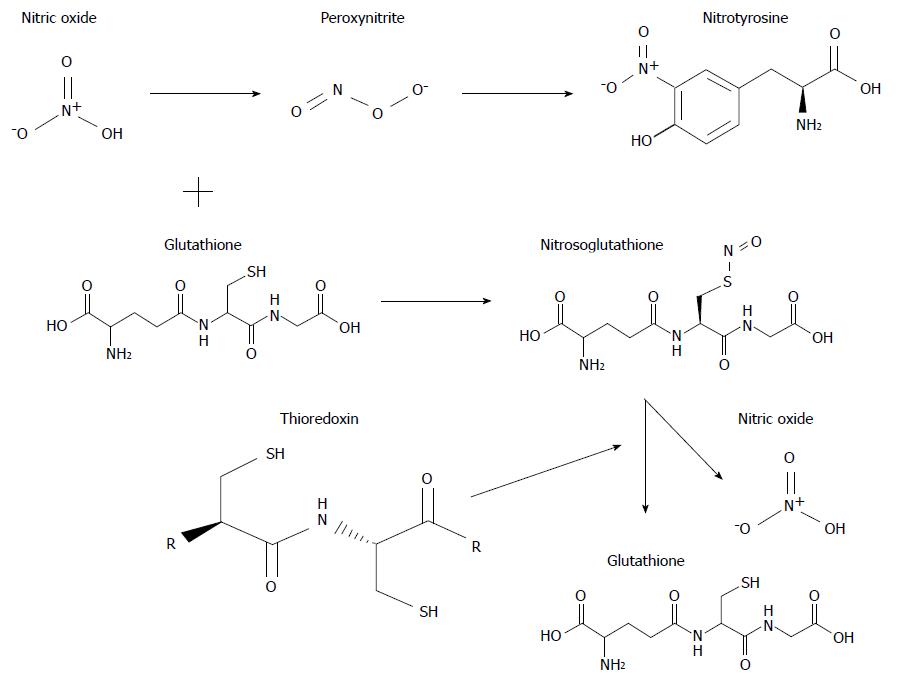
Figure 4 Structural formula and interaction of some important molecules involved in oxidative and nitrosative stress associated with chronic cholestasis.
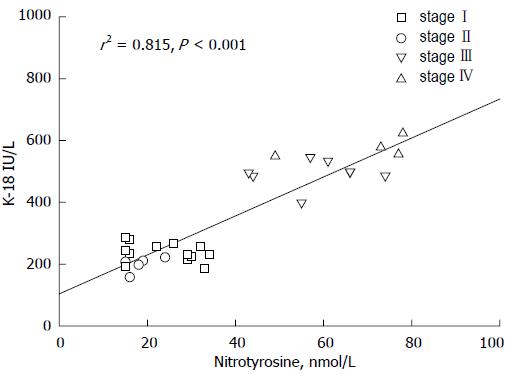
Figure 5 Link between nitric oxide derivatives and inflammatory products is linearly evidenced in this graph reporting the relationship between serum levels of nitrotyrosine and keratin-18.
Patients with primary biliary cirrhosis (n = 30) were divided in stages according to liver histology: stage I; stage II; stage III; stage IV. Data adapted from Grattagliano and Portincasa (unpublished). Nitrotyrosine and keratin-18 (K-18) concentrations measured as previously reported[82].
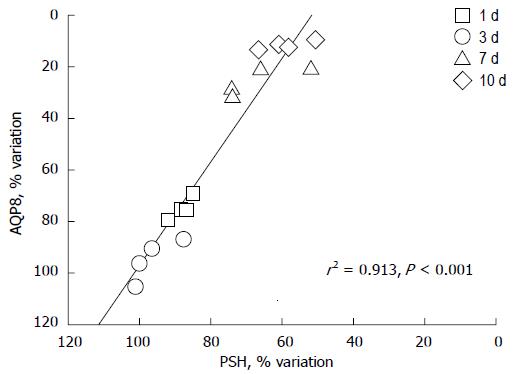
Figure 6 Aquaporin 8 is a specific water channel actively involved in the regulation of mitochondrial volume in the liver.
Protein sulfhydryls (PSH) are a diffuse category of proteins playing important roles in mitochondria, including structural proteins and respiratory complexes to channel pores. The graph depicts the correlation between densitometric values of aquaporin 8 (AQP8) immunoreactivity and PSH content in the liver mitochondria of sham-operated and bile duct ligated rats at different time-points. AQP8 expression varies in relation to the changes in PSH content (n = 16, r = +0.96, r2 = 0.913, P < 0.0001). N = 4 experiments per time point with 1 d, 3 d, 7 d and 10 d; BDL. Data adapted from Grattagliano and Portincasa (unpublished). Measurements performed as previously reported[65].
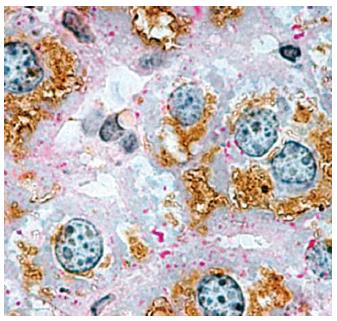
Figure 7 Immunohistochemical localization of aquaporin 8 in mouse hepatocytes.
Considerable aquaporin 8 (AQP8) immunoreactivity (brown staining) is seen within the cytoplasmic compartment of most hepatocytes. Periodic acid-Schiff (PAS) reactivity (red staining) is seen over glycogen granules. In rodents, hepatocyte AQP8 is expressed at multiple subcellular levels, including the apical (canalicular) plasma membrane, subapical vesicles, smooth endoplasmic reticulum, and mitochondria. The AQP8 channel features conductance to water, ammonia, and H2O2, and is suggested to be involved in primary bile secretion, ammonia detoxification, ureagenesis, and mitochondrial reactive oxygen species generation. Original magnification, × 1000. (micrograph from Ferri et al[22], reproduced with permission from Wiley Online Library).
- Citation: Grattagliano I, Calamita G, Cocco T, Wang DQH, Portincasa P. Pathogenic role of oxidative and nitrosative stress in primary biliary cirrhosis. World J Gastroenterol 2014; 20(19): 5746-5759
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5746.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5746