Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. May 14, 2014; 20(18): 5244-5251
Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5244
Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5244
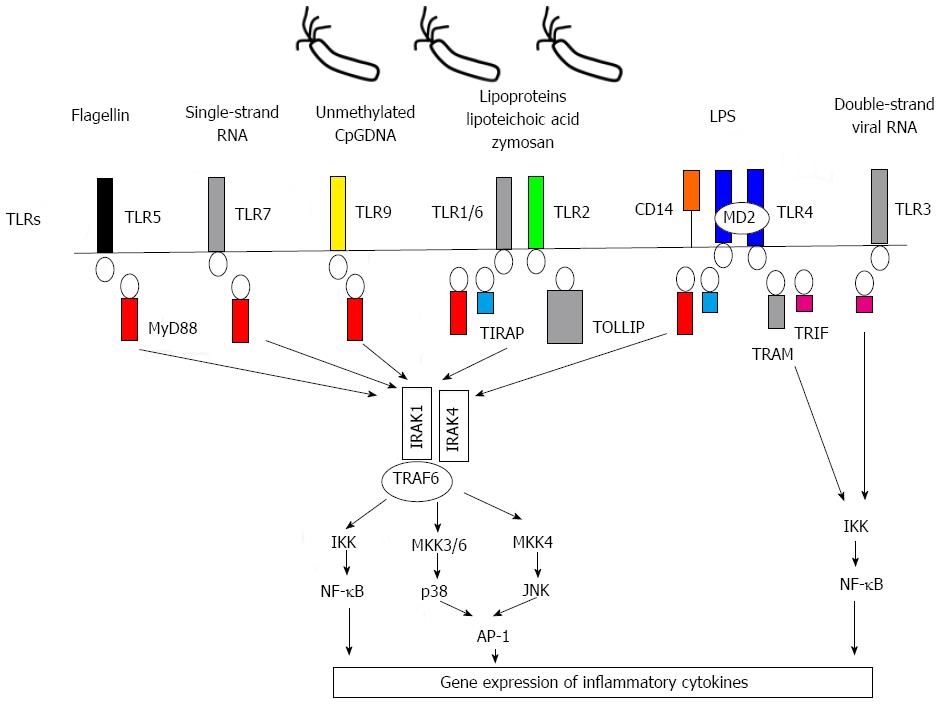
Figure 1 Intracellular signal propagation of human toll-like receptors and corresponding ligands in innate immunity.
Toll-like receptors (TLRs) and ligands in human are revealed in the figure. Individual TLRs recognize specific (pathogen-associated molecular patterns, PAMPs) or damage-associated molecular patterns (DAMPs) of corresponding ligands. TLR signaling is propagated by activation of its cytoplasmic TIR (Toll/IL-1R) domain and cooperation with various adaptor molecules, such as myeloid differentiation factor 88 (MyD-88), toll-interleukin 1 receptor (TIR) domain-containing adapter protein (TIRAP), toll interacting protein (TOLLIP), (IRAK), (TRAF), TIR domain-containing adapter inducing interferon (IFN)-beta (TRIF), and TRIF-related adapter molecule (TRAM). TLR signaling consists of two distinct pathways, MyD-88-dependent and MyD-88-independent: (1) MyD-88-dependent pathway: The MyD88 dependent pathway is down-stream of TLR1, TLR2, TLR4, TLR5, TLR6, TLR7 and TLR9. This pathway leads to the production of proinflammatory cytokines and is triggered by the association of activated TIR domain and MyD-88, recruitment of IRAK1, IRAK4 and TRAF6 to the TLR-MyD-88 complex and consequent phosphorylation of IRAK1 and TRAF6. The signal is propagated by this phosphorylated adaptor molecular complex to the down-stream MAP kinases-AP1 and IKK complex-NF-κB; (2) MyD88-independent pathway: This is associated with the induction of INF-beta mediated by TLR3 or TLR4 activation. Intracellular signaling via the MyD-88 independent pathway is propagated by the action of TRIF and TRAM as adaptor molecules. The signal consequently activates the IKK pathway to produce INF-beta.
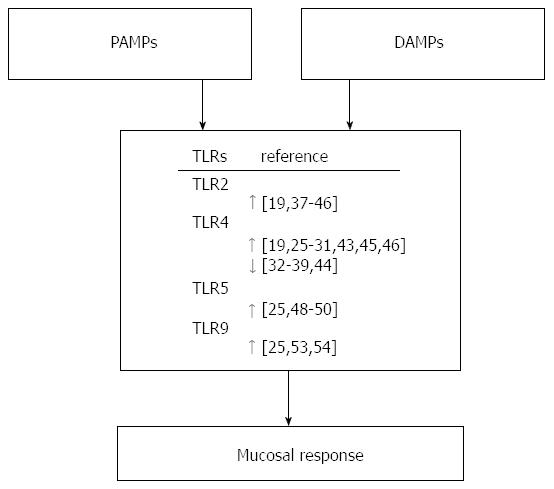
Figure 2 Role of toll-like receptors in Helicobacter pylori induced innate immunity.
Toll-like receptors (TLRs) can sense structurally conserved molecules (pathogen-associated molecular patterns, PAMPs) as well as damage-associated molecular patterns (DAMPs) produced under stress conditions. These recognition systems direct the wide-ranging responsiveness of TLRs not only to foreign agents, but also to internal organisms denatured by inflammation.
-
Citation: Uno K, Kato K, Shimosegawa T. Novel role of toll-like receptors in
Helicobacter pylori - induced gastric malignancy. World J Gastroenterol 2014; 20(18): 5244-5251 - URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5244.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5244