Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 28, 2014; 20(16): 4806-4810
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4806
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4806
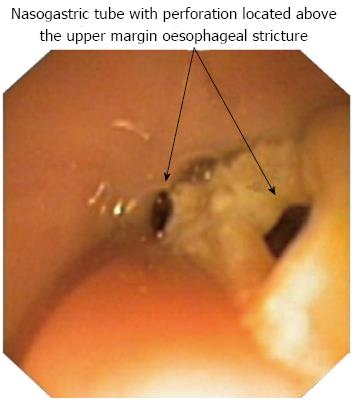
Figure 1 Insertion of nasogastric tube with perforations located above the stricture margin.
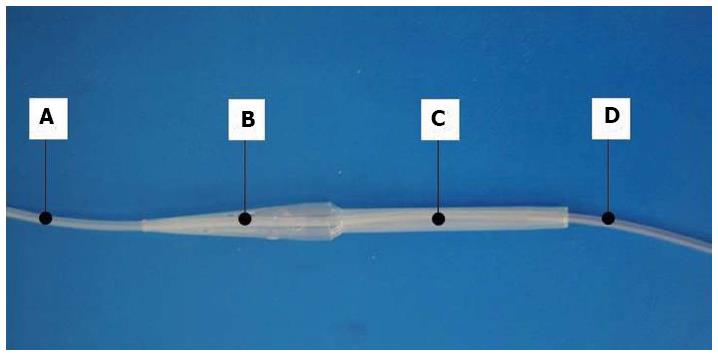
Figure 2 Newly developed nasogastric tube.
A perforated nasogastric oesophageal closure protecting tube made of a polyamide polymer, with a double lumen and varying diameter, was developed at the Children’s Memorial Health Institute. A: Proximal part of the 8 Fr nasogastric tube; B: Conical, perforated segment setting the tube above the stenosis; C: Portion of the tube located within the stenosis and preventing it from narrowing; D: Distal end of the 8 Fr nasogastric tube to be introduced into the stomach. Liquid diet can be administered into the stomach directly by the nasogastric tube, while oral liquids and saliva can drain through the perforation in the conical portion (B) into the portion located within the stenosis (C), and further into healthy oesophagus below the stenosis. The technology of tube construction, manufacturing and prototype tube production were performed by Balton Ltd. The tube has been registered at the Polish Patent Office (No. P.399031)[9].
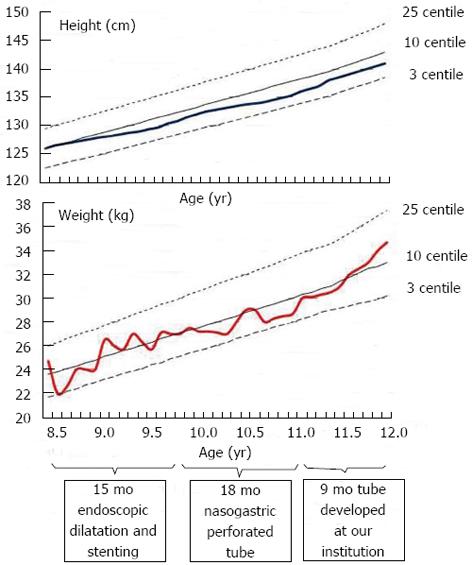
Figure 3 Patient’s grow charts.
Height and weight of the patient during three phases of therapy: oesophageal dilatation and stenting (15 mo); nasogastric tube with perforation for saliva and fluid passage (18 mo); double lumen, varying diameter nasogastric tube developed at our institution (9 mo).
- Citation: Woynarowski M, Dądalski M, Wojno V, Teisseyre M, Szymczak M, Chyżyńska A, Hurkała L, Płowiecki E, Kmiotek J. Nasogastric tube as protection for recurrent oesophageal stricture: A case report. World J Gastroenterol 2014; 20(16): 4806-4810
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4806.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4806