Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 7, 2013; 19(5): 673-681
Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.673
Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.673
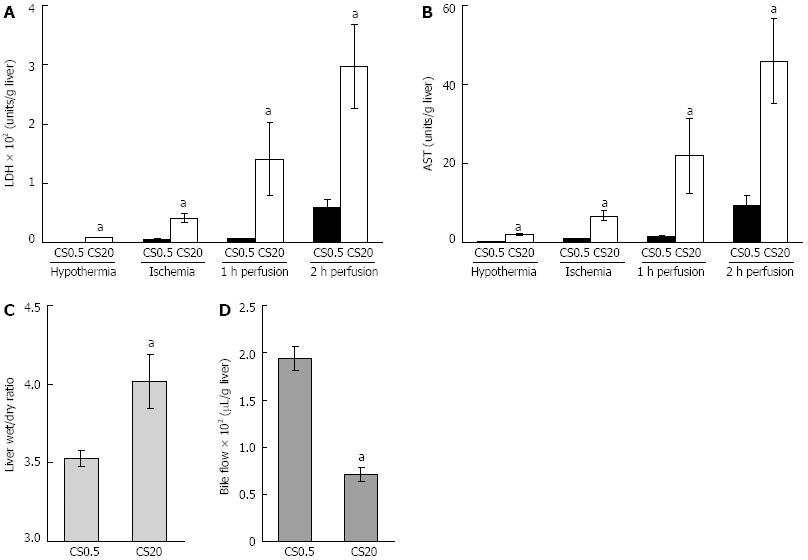
Figure 1 Liver function following cold storage, warm ischemia and perfusion.
A, B: Liver damage was assessed by measuring lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) in media from control livers after 0.5 h of cold storage (CS0.5) and from the livers after 20 h of extended cold storage (CS20). LDH and AST were measured in media flushed from livers following cold storage, in media flushed from livers following 25 min of warm ischemia and in media at 1 and 2 h of warm perfusion; C, D: Liver health was assessed by liver swelling (liver wet to dry ratio) after 2 h of perfusion and bile flow over 2 h of perfusion. Significantly different from the controls (mean ± SE, n = 5, aP < 0.05).
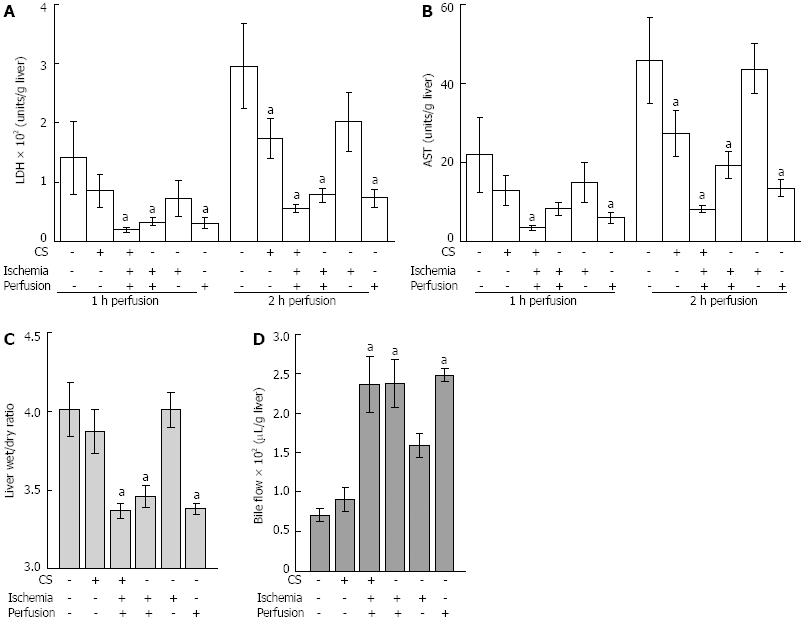
Figure 2 The effect of desferrioxamine added during cold storage, warm ischemia and/or perfusion on liver function following 20 h of cold storage.
A,B: Desferrioxamine was added (indicated by +) to cold storage (CS), warm ischemia and perfusion media. Liver damage was assessed by measuring lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) in media from livers during perfusion at 1 and 2 h; C, D: Liver health was assessed by liver swelling (liver wet to dry ratio) after 2 h of perfusion and bile flow over 2 h of perfusion. Data for extended (20 h) CS are shown. Significantly different from 20 h of CS (mean ± SE, n = 5, aP < 0.05).
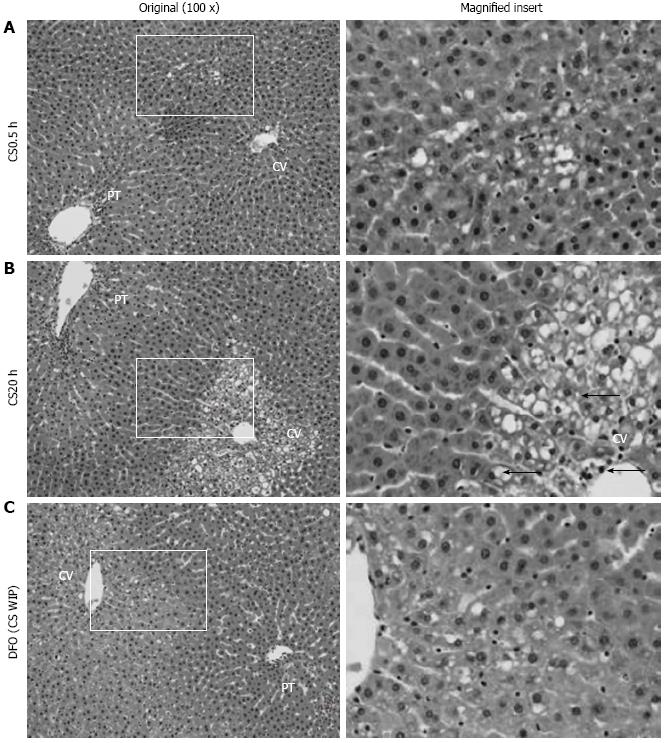
Figure 3 Histopathological appearance of livers following cold storage, warm ischemia and perfusion and the effect of desferrioxamine.
A: The control liver shows some early vacuolization in zone 2 following 0.5 h of cold storage (CS0.5) (magnified insert); B: Following 20 h of cold storage (CS20), the liver shows marked vacuolization and nuclear pyknosis in zones 3 as indicated by arrows (←, magnified insert); C: Following 20 h of CS with desferrioxamine (DFO) in CS, warm ischemia and perfusion media (CS WIP), the liver shows mild vacuolization in zone 3 (magnified insert). Hematoxylin and eosin staining and the original magnification was 100 ×. PT: Portal tract; CV: Central vein.
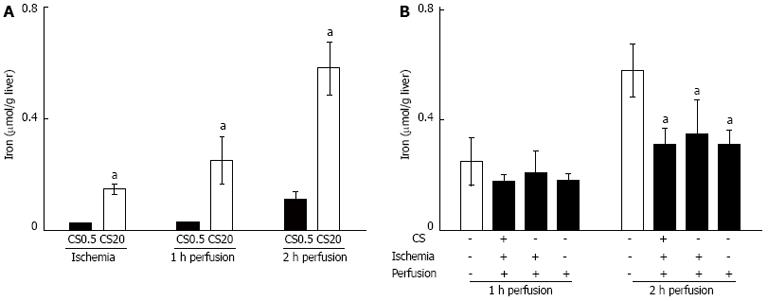
Figure 4 Iron release during warm ischemia and perfusion and effect of desferrioxamine on iron release.
A: Iron release was measured in media after warm ischemia (WI) and at 1 and 2 h of perfusion from the control livers after 0.5 h of cold storage (CS0.5) and from the livers after 20 h of extended cold storage (CS20). Significantly different from the controls (mean ± SE, n = 5, aP < 0.05); B: Iron release was measured in media at 1 and 2 h of perfusion from livers following 20 h of cold storage (CS). Addition of desferrioxamine (DFO) to CS, WI and perfusion media is indicated by (+). Significantly different from 20 h of CS with no DFO (mean ± SE, n = 5, aP < 0.05).
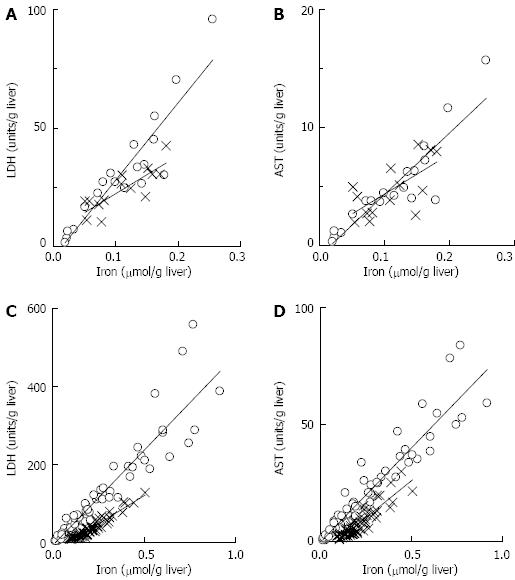
Figure 5 Relationship between presence of iron and liver damage.
Following ischemia: A: The relationships between lactate dehydrogenase (LDH) and iron in the absence of desferrioxamine (DFO) (circles) and the presence of DFO (crosses) were described by y = 327x - 5 (n = 19, r2 = 0.84) and y = 162x + 6 (n = 14, r2 = 0.69) respectively; B: The relationships between aspartate aminotransferase (AST) and iron in the absence of DFO (circles) and the presence of DFO (crosses) were described by y = 52x - 0.9 (n = 19, r2 = 0.81) and y = 33x + 1 (n = 14, r2 = 0.44); C: During perfusion, samples collected at 0.5 h, 1 h, 1.5 h and 2 h were analysed. The relationships between LDH and iron in the absence of DFO (circles) and the presence of DFO (crosses) were described by y = 487x - 8.1 (n = 76, r2 = 0.88) and y = 291x - 24.4 (n = 56, r2 = 0.91) respectively; D: The relationships between AST and iron in the absence of DFO (circles) and the presence of DFO (crosses) were described by y = 81x - 0.5 (n = 76, r2 = 0.90) and y = 62x - 5.6 (n = 56, r2 = 0.80) respectively. All correlation coefficients were significant (P < 0.05), and all slopes for media containing iron were significantly different (P < 0.05) from the equivalent treatment without DFO.
- Citation: Arthur PG, Niu XW, Huang WH, DeBoer B, Lai CT, Rossi E, Joseph J, Jeffrey GP. Desferrioxamine in warm reperfusion media decreases liver injury aggravated by cold storage. World J Gastroenterol 2013; 19(5): 673-681
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.673