Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 14, 2013; 19(34): 5738-5749
Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5738
Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5738
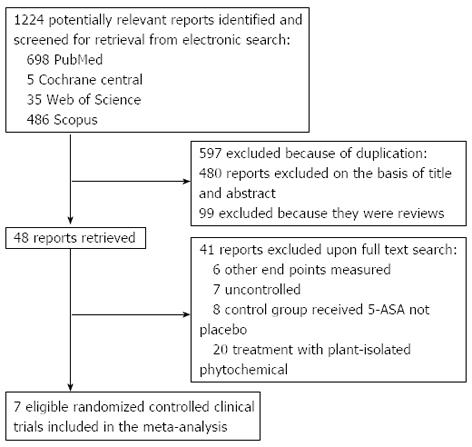
Figure 1 Flow diagram of the study selection process.
ASA: Aminosalicylic acid.
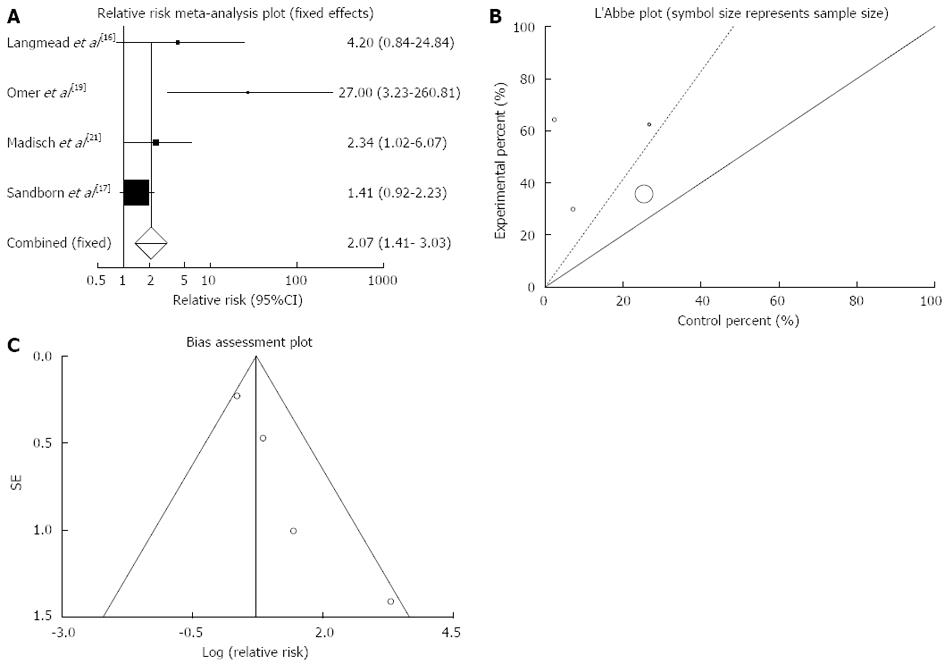
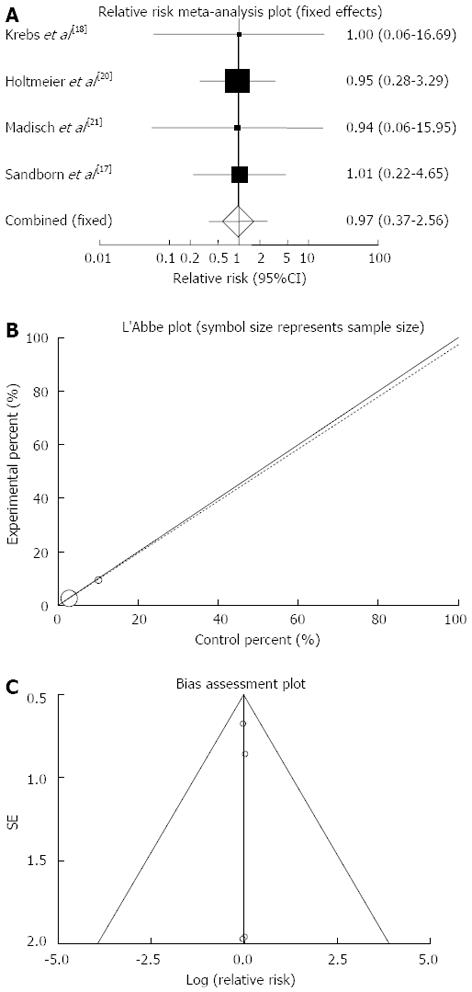
Figure 2 Individual and pooled relative risk (A), heterogeneity indicators (B) and publication bias indicators (C) for the outcome of “clinical remission” in the studies considering herbal medicines comparing to placebo therapy in inflammatory bowel disease patients.
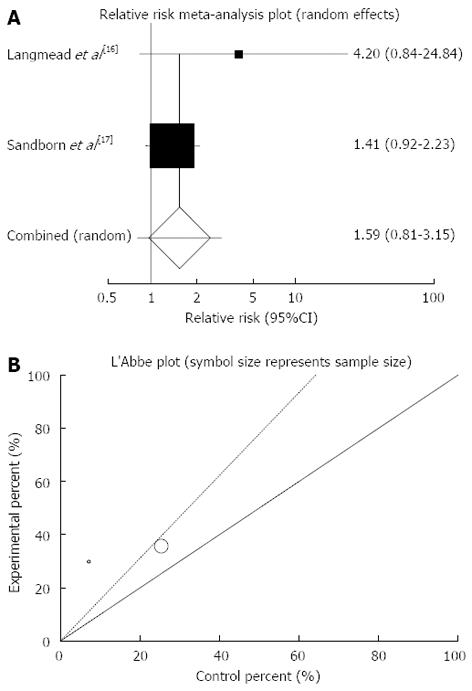
Figure 3 Individual and pooled relative risk (A) and heterogeneity indicators (B) for the outcome of “clinical remission” in the studies considering herbal medicines comparing to placebo therapy in ulcerative colitis patients.
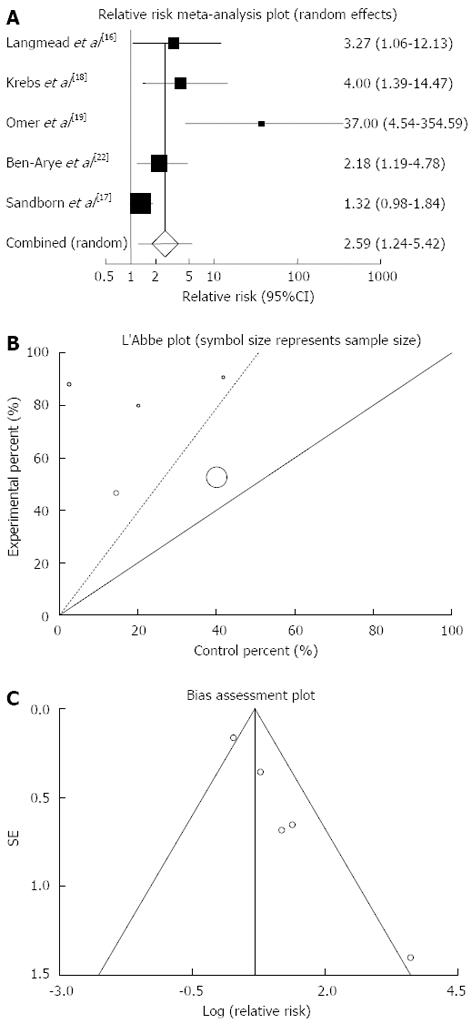
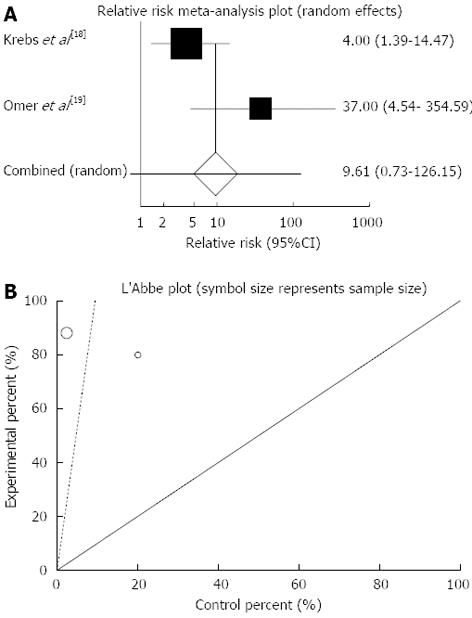
Figure 4 Individual and pooled relative risk (A), heterogeneity indicators (B) and publication bias indicators (C) for the outcome of “clinical response” in the studies considering herbal medicines comparing to placebo therapy in inflammatory bowel disease patients.
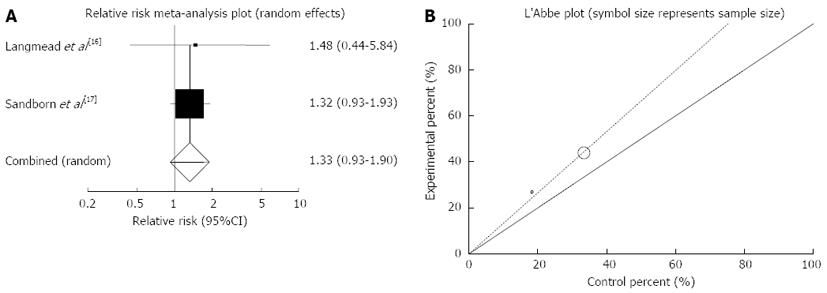
Figure 5 Individual and pooled relative risk (A) and heterogeneity indicators (B) for the outcome of “clinical response” in the studies considering herbal medicines comparing to placebo therapy in Crohn’s disease patients.
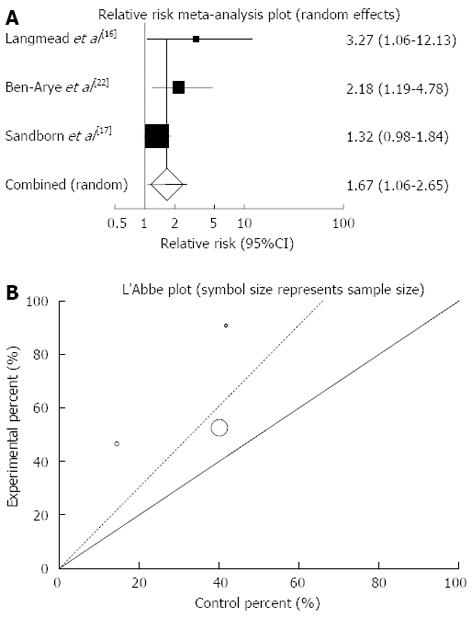
Figure 6 Individual and pooled relative risk (A) and heterogeneity indicators (B) for the outcome of “clinical response” in the studies considering herbal medicines comparing to placebo therapy in ulcerative colitis patients.
Figure 7 Individual and pooled relative risk (A) and heterogeneity indicators (B) for the outcome of “endoscopic remission” in the studies considering herbal medicines comparing to placebo therapy in inflammatory bowel disease (ulcerative colitis) patients.
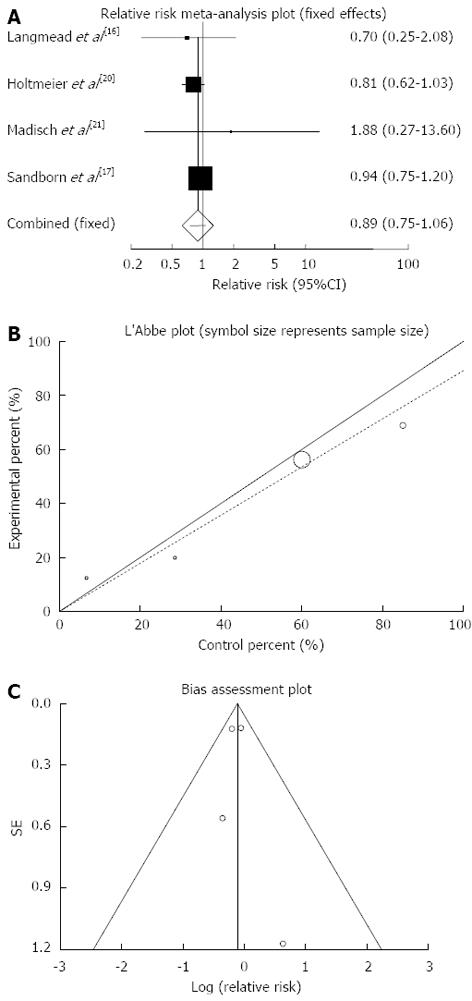
Figure 8 Individual and pooled relative risk (A), heterogeneity indicators (B) and publication bias indicators (C) for the outcome of “any adverse events” in the studies considering herbal medicines comparing to placebo therapy in inflammatory bowel disease patients.
Figure 9 Individual and pooled relative risk (A), Heterogeneity indicators (B) and publication bias indicators (C) for the outcome of “serious adverse events” in the studies considering herbal medicines comparing to placebo therapy in inflammatory bowel disease patients.
- Citation: Rahimi R, Nikfar S, Abdollahi M. Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: A meta-analysis. World J Gastroenterol 2013; 19(34): 5738-5749
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5738.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5738