Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 7, 2013; 19(33): 5485-5492
Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5485
Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5485
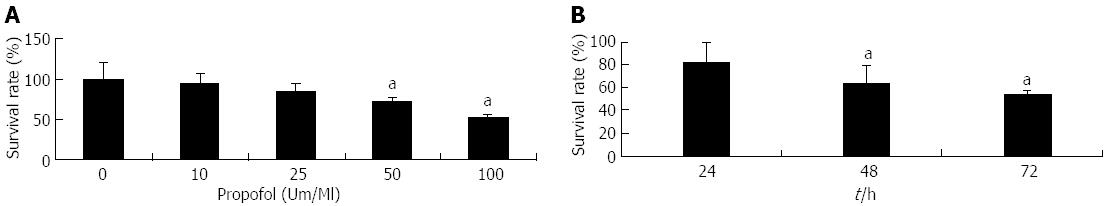
Figure 1 Evaluation by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay of pancreatic cancer MIA-PaCa-2 cell viability after propofol pretreatment.
A: Cells were either untreated or treated with 10-100 μmol/mL propofol for 72 h; B: Cells treated with 100 μmol/mL propofol for 24, 48, or 72 h. aP < 0.05 vs control group.
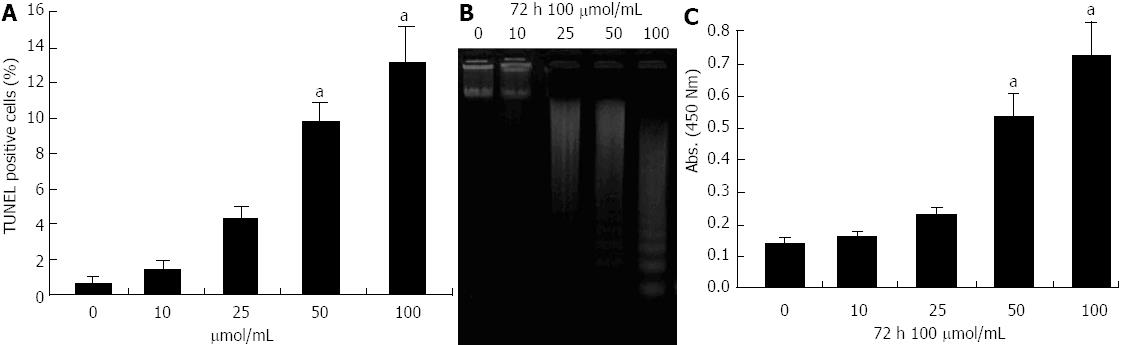
Figure 2 Evaluation of apoptosis of pancreatic cancer MIA-PaCa-2 cells after propofol treatment using terminal transferase dUTP nick-end labeling, DNA ladder and enzyme-linked immunosorbent assays.
A: Propofol-induced apoptotic cell death by terminal transferase dUTP nick-end labeling (TUNEL) after 72 h of 10-100 μmol/L propofol; B: DNA ladder indicative of apoptosis in pancreatic cancer cells treated with 10-100 μmol/mL propofol for 72 h. C: Propofol-induced apoptosis measured by enzyme-linked immunosorbent assay after 72 h of 10-100 μmol/L propofol. aP < 0.05 vs control group.
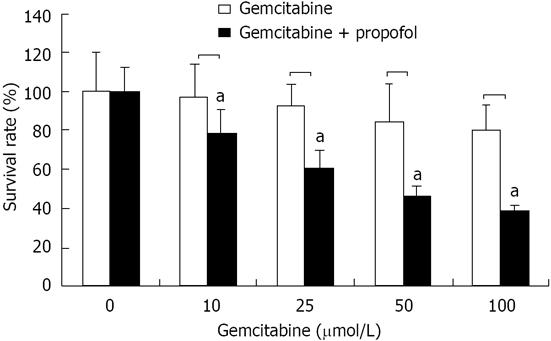
Figure 3 Cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay of pancreatic cancer MIA-PaCa-2 cells after propofol pretreatment.
MIA-PaCa-2 cells pretreated with propofol (50 μmol/mL) for 24 h followed by coincubation with gemcitabine (10, 25, 50 and 100 μmol/L) for 72 h were analyzed for viable cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. Viable cells were evaluated relative to gemcitabine-treated controls and interpreted as % viable cells. Data are averages of four to five independent experiments. aP < 0.05 vs control group.
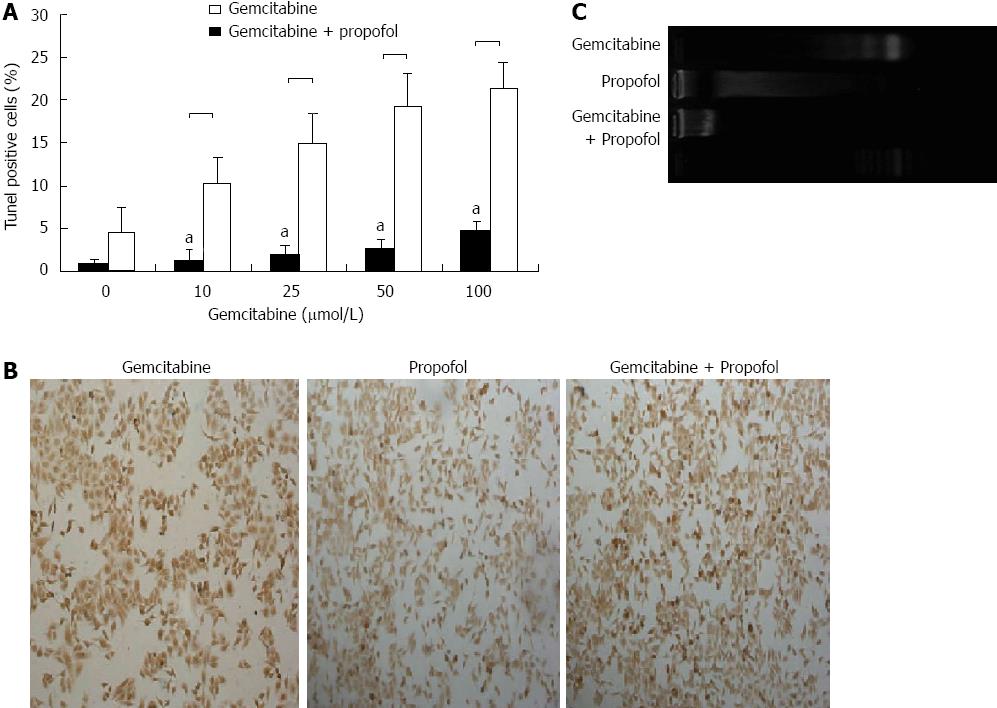
Figure 4 Evaluation of apoptosis by terminal transferase dUTP nick-end labeling and DNA ladder assays in pancreatic cancer MIA-PaCa-2 cells after propofol pretreatment.
A: Sensitization of pancreatic tumor MIA-PaCa-2 cells to propofol- and/or gemcitabine-induced apoptosis measured by terminal transferase dUTP nick-end labeling (TUNEL) assay after 24 h of pretreatment with propofol (50 μmol/mL), gemcitabine (0-100 μmol/L), or propofol and gemcitabine combined for 72 h. Increased apoptosis was evident in the combination treatment group relative to individual treatment groups. aP < 0.05 vs propofol and gemcitabine combined group; B: Representative TUNEL image of MIA-PaCa-2 cells pretreated with propofol (50 μmol/mL) for 24 h followed by coincubation with gemcitabine (50 μmol/L) for 72 h; C: Representative DNA ladder image of MIA-PaCa-2 cells pretreated with propofol (50 μmol/mL) for 24 h followed by coincubation with gemcitabine (50 μmol/L) for 72 h.
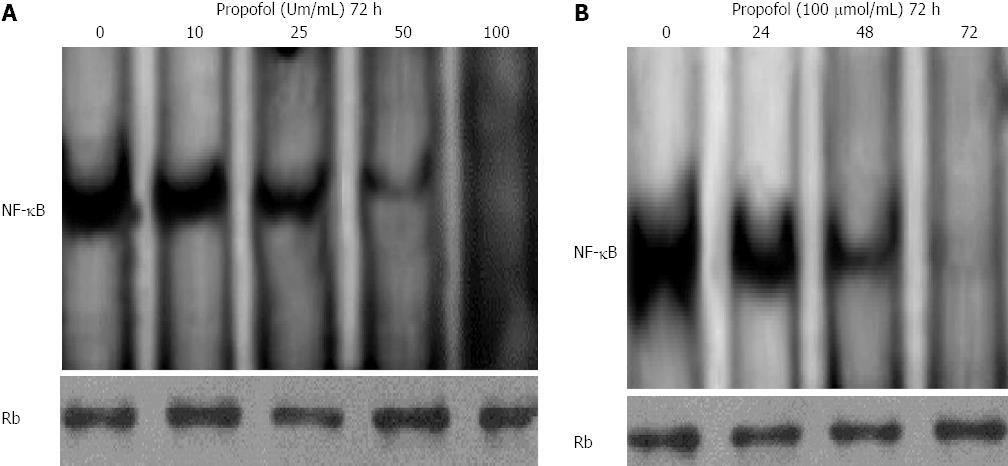
Figure 5 Propofol inhibits constitutively active nuclear factor-κB in MIA-PaCa-2 cells.
A: MIA-PaCa-2 cells were treated with 0, 10, 25, 50, or 100 μmol/mL propofol for 72 h and nuclear extracts were probed for nuclear factor-κB (NF-κB) binding to a DNA consensus sequence. Results of NF-κB DNA-binding activity by electrophoretic mobility shift assay (EMSA); B: MIA-PaCa-2 cells were treated with 100 μmol/mL propofol for 24, 48, or 72 h and nuclear extracts were probed for NF-κB binding to a DNA consensus sequence. Results of NF-κB DNA-binding activity by EMSA. Retinoblastoma protein in the nuclear extract was used as a loading control.
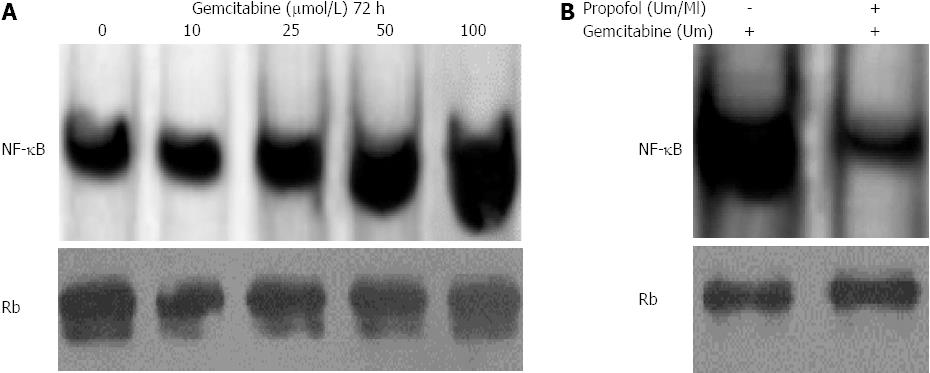
Figure 6 Propofol abrogates gemcitabine-induced nuclear factor-κB in MIA-PaCa-2 cells.
A: MIA-PaCa-2 cells were exposed to 10-100 μmol/L gemcitabine for 72 h. Nuclear extracts were analyzed by electrophoretic mobility shift assay (EMSA); B: Propofol abrogated gemcitabine induced nuclear factor-κB (NF-κB) in MIA-PaCa-2 cells exposed to 100 μmol/L/mL propofol for 24 h followed by incubation with 100 μmol/L gemcitabine. Nuclear extracts were harvested at 72 h and analyzed by EMSA. Propofol pretreatment downregulated gemcitabine-induced NF-κB with 100 μmol/L gemcitabine.
-
Citation: Du QH, Xu YB, Zhang MY, Yun P, He CY. Propofol induces apoptosis and increases gemcitabine sensitivity in pancreatic cancer cells
in vitro by inhibition of nuclear factor-κB activity. World J Gastroenterol 2013; 19(33): 5485-5492 - URL: https://www.wjgnet.com/1007-9327/full/v19/i33/5485.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i33.5485