Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 14, 2012; 18(46): 6790-6800
Published online Dec 14, 2012. doi: 10.3748/wjg.v18.i46.6790
Published online Dec 14, 2012. doi: 10.3748/wjg.v18.i46.6790
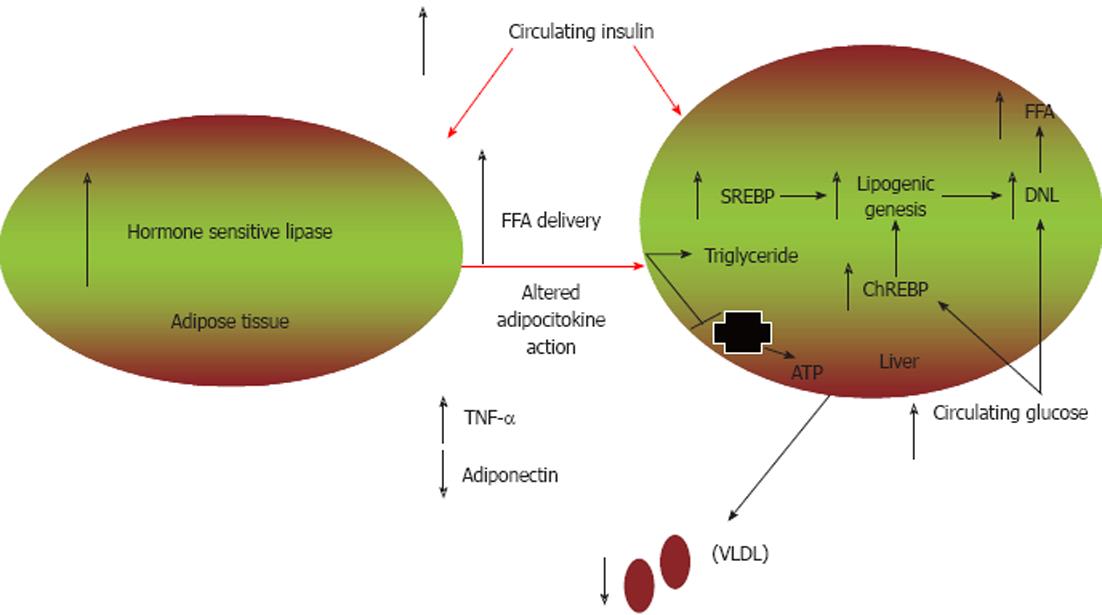
Figure 1 Mechanisms contributing to glucose and lipid dysmetabolism.
In the setting of insulin resistance, there is increased adipose tissue hormone-sensitive lipase activity that results in enhanced lipolysis and increased non-esterified fatty acid (NEFA) delivery to the liver. NEFAs are preferentially esterified to triglycerides. Additionally, hyperinsulinaemia leads to increased sterol regulatory element patients with NAFLD binding protein (SREBP) expression, resulting in increased de novo lipogenesis (DNL) and decreased fatty acid oxidation. Carbohydrate response element-binding protein (ChREBP) is induced by hyperglycaemia and leads to further increases in DNL. Decreased hepatic lipid transport may also occur, in part via altered synthesis of apolipoprotein B, leading to decreased very low-density lipoprotein (VLDL) production. TNF-α: Tumour necrosis factor-α; FFA: Free fatty acid.
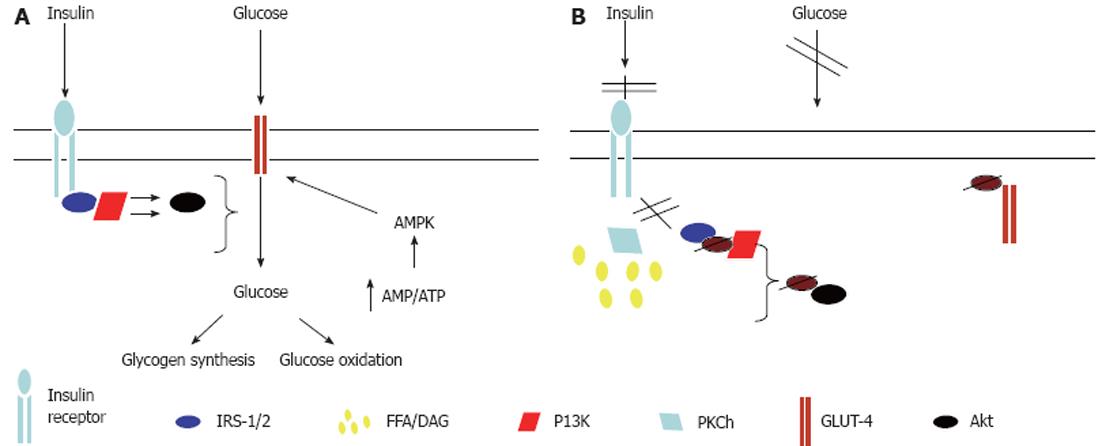
Figure 2 Mechanisms for free fatty acid-induced skeletal muscle insulin resistance.
A: Normal glucose uptake into skeletal muscle occurs via binding of insulin to the insulin receptor, resulting in receptor autophosphorylation and subsequent binding and tyrosine phosphorylation of insulin receptor substrate (IRS)-1. Subsequently, phosphatidylinositol 3-kinase (PI3K) is activated and results in downstream activation of protein kinase B, leading to glucose transporter (GLUT)-4 translocation to the myocyte plasma membrane and glucose uptake into the cell; B: Increased intramyocellular lipid content leads to the activation of protein kinase Ch (PKCh) which results in serine phosphorylation of IRS-1, thereby inhibiting its tyrosine phosphorylation. This prevents the activation of PI3K and GLUT-4 translocation to the cell surface. Thus glucose entry into the cell is inhibited. AMPK: AMP-activated protein kinase; DAG: Diacylglycerol; FFA: Free fatty acid; Akt: Protein Kinase B.
- Citation: Finelli C, Tarantino G. Have guidelines addressing physical activity been established in nonalcoholic fatty liver disease? World J Gastroenterol 2012; 18(46): 6790-6800
- URL: https://www.wjgnet.com/1007-9327/full/v18/i46/6790.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i46.6790