Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 21, 2012; 18(43): 6290-6301
Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6290
Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6290
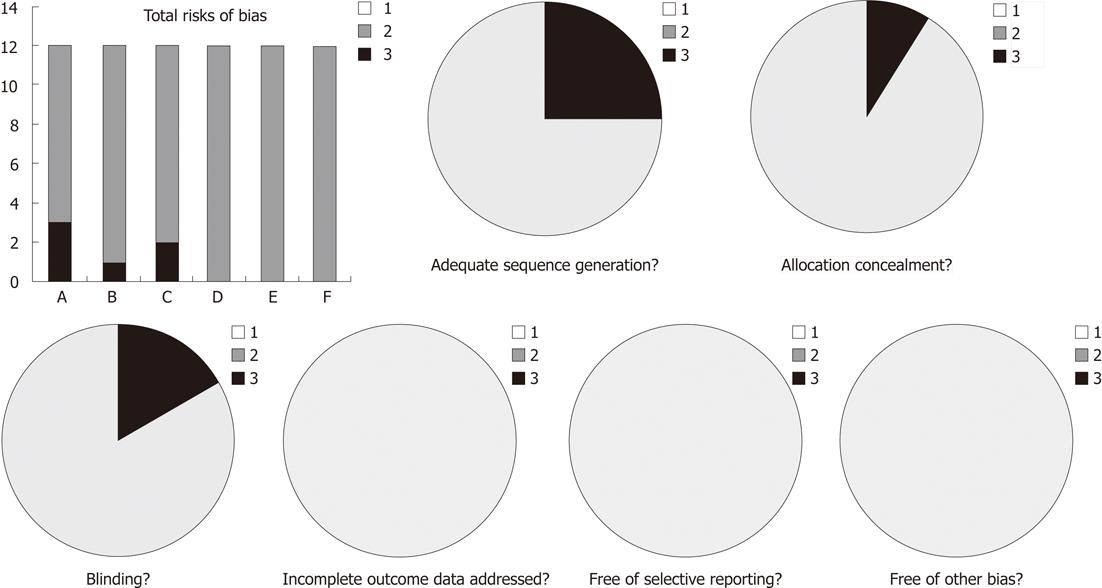
Figure 1 Risk of bias in included trials.
A: Adequate sequence generation; B: Allocation concealment; C: Blinding; D: Incomplete outcome data addressed; E: Free of selective reporting; F: Free of other bias. 1: No (high risk of bias); 2: Unclear; 3: Yes (low risk of bias).
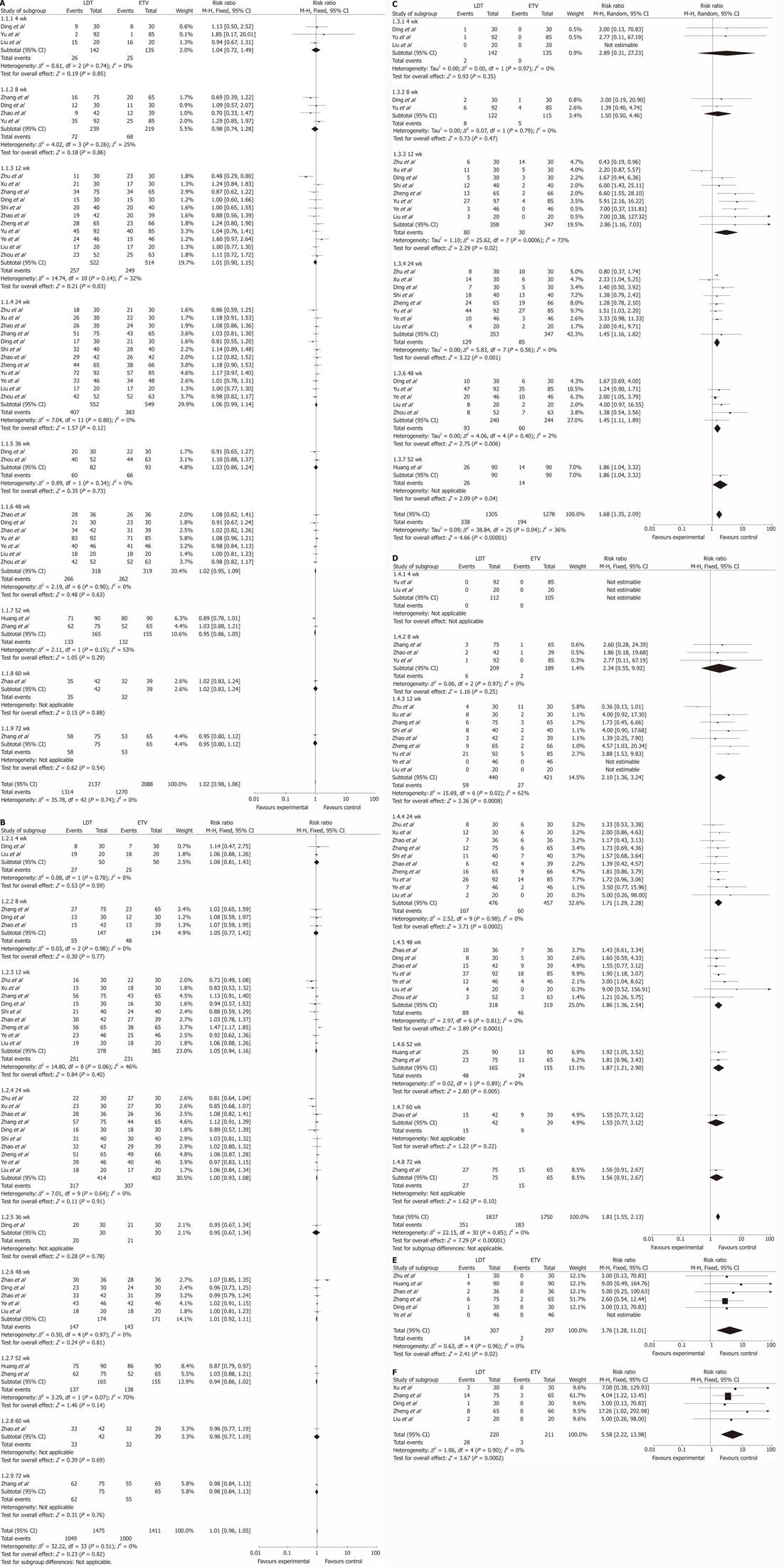
Figure 2 Meta-analysis of the two groups.
A: Hepatitis B virus DNA undetectability; B: Alanine aminotransferase normalization; C: Hepatitis B e antigen (HBeAg) loss; D: HBeAg seroconversion; E: Drug-resistance; F: Increased creatine kinase (CK). ETV: Entacavir; LDT: Telbivudine.
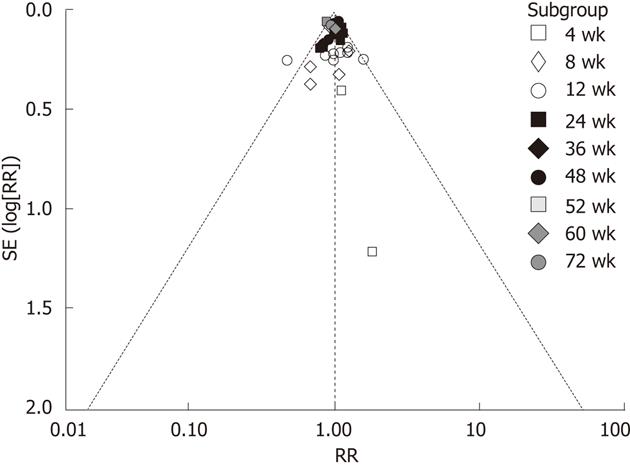
Figure 3 Funnel plots of the two groups in hepatitis B virus DNA undetectability.
RR: Relative risk;
- Citation: Su QM, Ye XG. Effects of telbivudine and entecavir for HBeAg-positive chronic hepatitis B: A meta-analysis. World J Gastroenterol 2012; 18(43): 6290-6301
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6290.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6290