Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 14, 2012; 18(42): 6005-6017
Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6005
Published online Nov 14, 2012. doi: 10.3748/wjg.v18.i42.6005
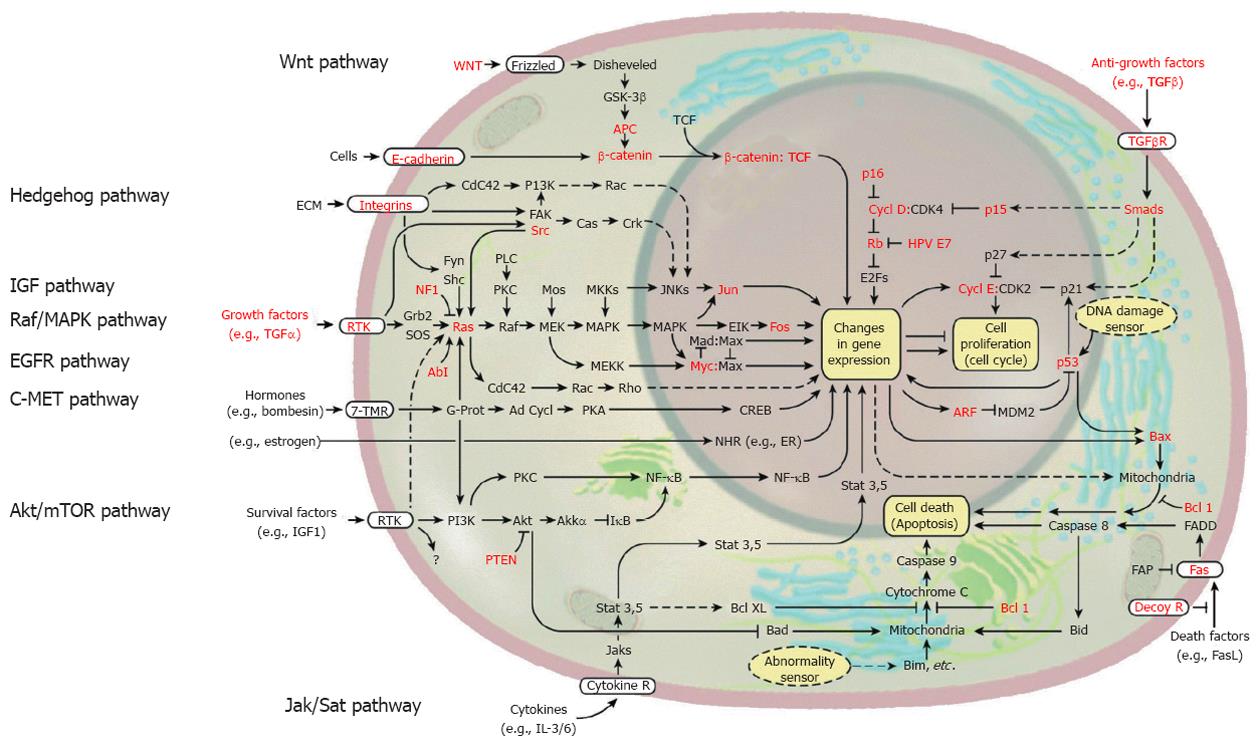
Figure 1 Signal transduction in solid cancer cells including hepatocellular carcinoma.
Some of the genes known to be functionally altered are highlighted in red. These signaling pathways, including growth factor pathway, Wnt pathway, Hedgehog pathway, Akt/mammalian target of rapamycin (mTOR) pathway, and Jak/Stat pathway, can be a molecular targets for treatment of hepatocellular carcinoma. (Cited and modified from Hanahan et al[10] with permission.) EGFR: Epidermal growth factor receptor; TGF: Transforming growth factor; IGF: Insulin-like growth factor; MAPK: Mitogen-activated protein kinase; PI3K: Phosphoinositide 3-kinase; ERK: Extracellular signaling-regulated kinase; NF-κB: Nuclear factor-kappa B; IL: Interleukin.
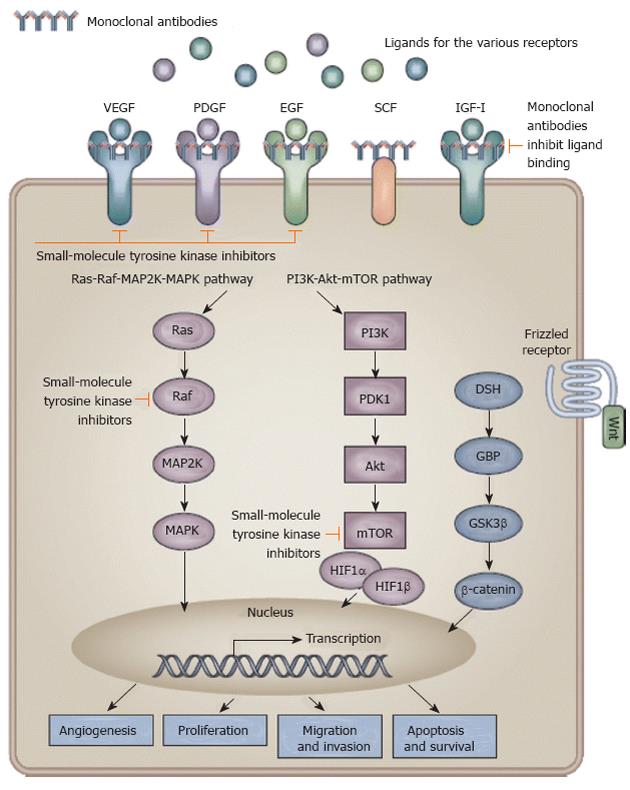
Figure 2 Signaling pathways and potential drug targets to inhibit hepatocarcinogenesis.
Activation of receptor tyrosine kinases by their ligands activates downstream signaling pathways with effects on angiogenesis, proliferation, migration and invasion, and apoptosis or survival of cells. Monoclonal antibodies inhibit ligand binding to the receptor and small-molecule tyrosine kinase inhibitors inhibit propagation of the downstream signal. (Cited from Spangenberg et al[11] with permission.) IGF: Insulin-like growth factor; MAPK: Mitogen-activated protein kinase; PI3K: Phosphoinositide 3-kinase; EGF: Epidermal growth factor; VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; mTOR: Mammalian target of rapamycin; HIF: Hypoxia-inducible factor; SCF: Stem cell factor.
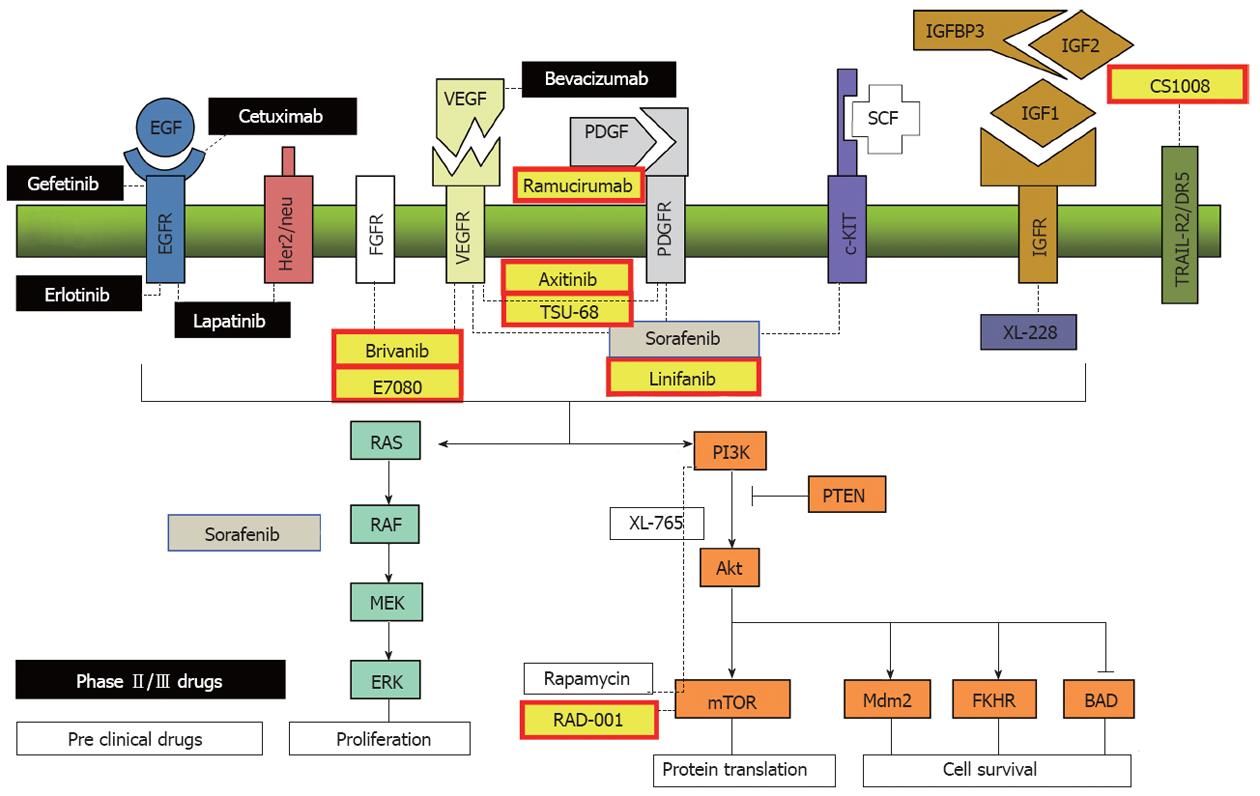
Figure 3 Target molecular and targeted agents under development.
Monoclonal antibodies (VEGF: bevacizumab, EGFR: cetuxinab), tyrosine kinase inhibitors (VEGFR: sorafenib, brivanib, linifanib, axitinib, TSU-68; FGFR: E7080, brivanib), EGFR: erlotinib, lapatinib), serine/threonine kinase inhibitors (Raf: sorafenib, mTOR: rapamycin and everolimus) and agonistic ligand of TRAIL-R2/DR5 (CS1008). (Cited and modified from Villanueva et al[12] with permission.) IGF: Insulin-like growth factor; PI3K: Phosphoinositide 3-kinase; EGF: Epidermal growth factor; VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; mTOR: Mammalian target of rapamycin; PTEN: Phosphatase and tensin homolog; SCF: Stem cell factor; FGFR: Fibroblast growth factor receptor.
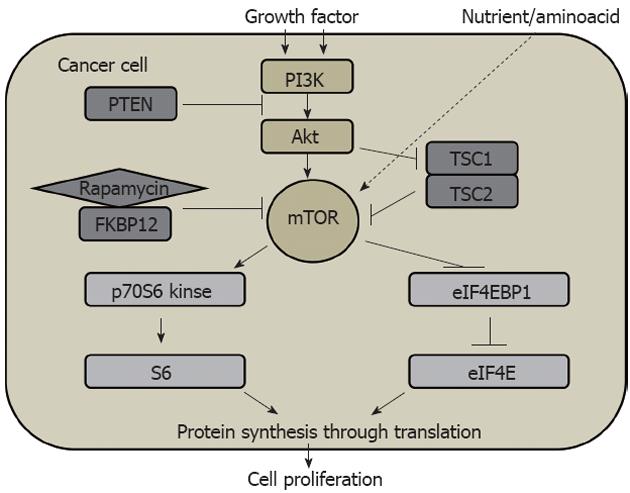
Figure 4 Phosphoinositide 3-kinase/Akt/mammalian target of rapamycin signaling pathway in cell proliferation in solid cancer.
PI3K: Phosphoinositide 3-kinase; mTOR: Mammalian target of rapamycin; PTEN: Phosphatase and tensin homolog; TSC: Tuberous sclerosis; FKBP12: FK506-binding protein 12.
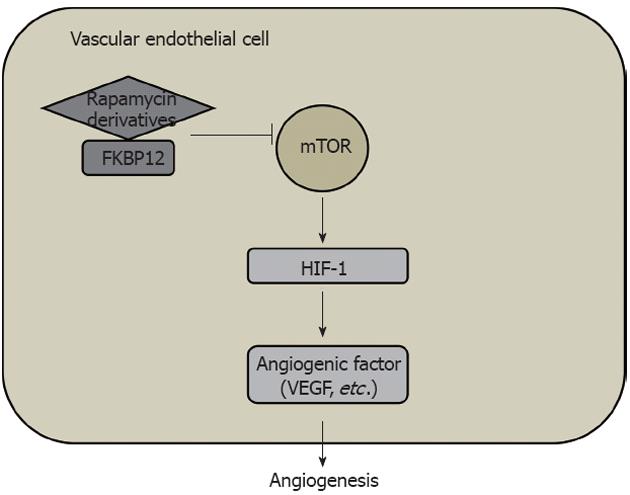
Figure 5 Mammalian target of rapamycin/hypoxia-inducible factor-1/vascular endothelial growth factor signaling pathway in angiogenesis in solid cancer.
HIF: Hypoxia-inducible factor; VEGF: Vascular endothelial growth factor; mTOR: Mammalian target of rapamycin; FKBP12: FK506-binding protein 12.
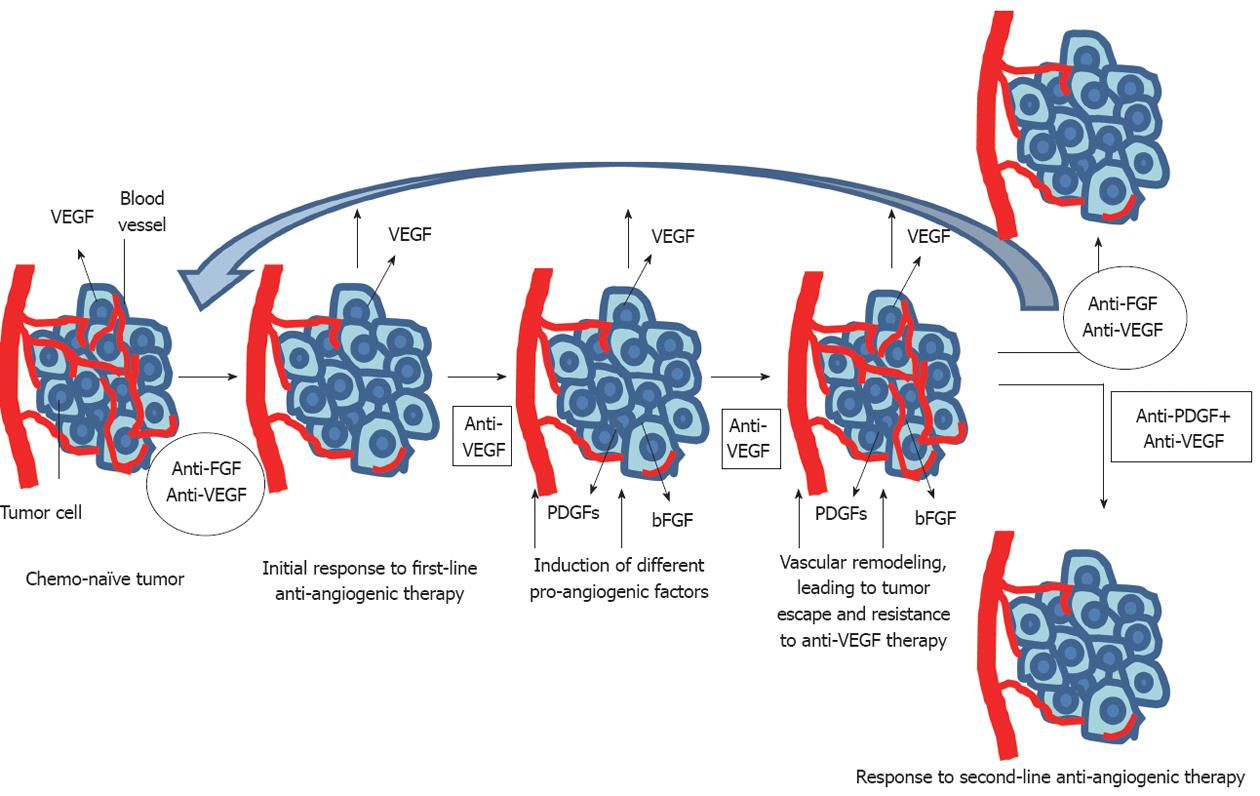
Figure 6 Brivanib may be effective for the failure or resistance of first line anti-angiogenic therapy for vascular endothelial growth factor.
In addition, there is a possibility that anti-FGF agents can be first line anti-angiogenic agents. FGF: Fibroblast growth factor; VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor.
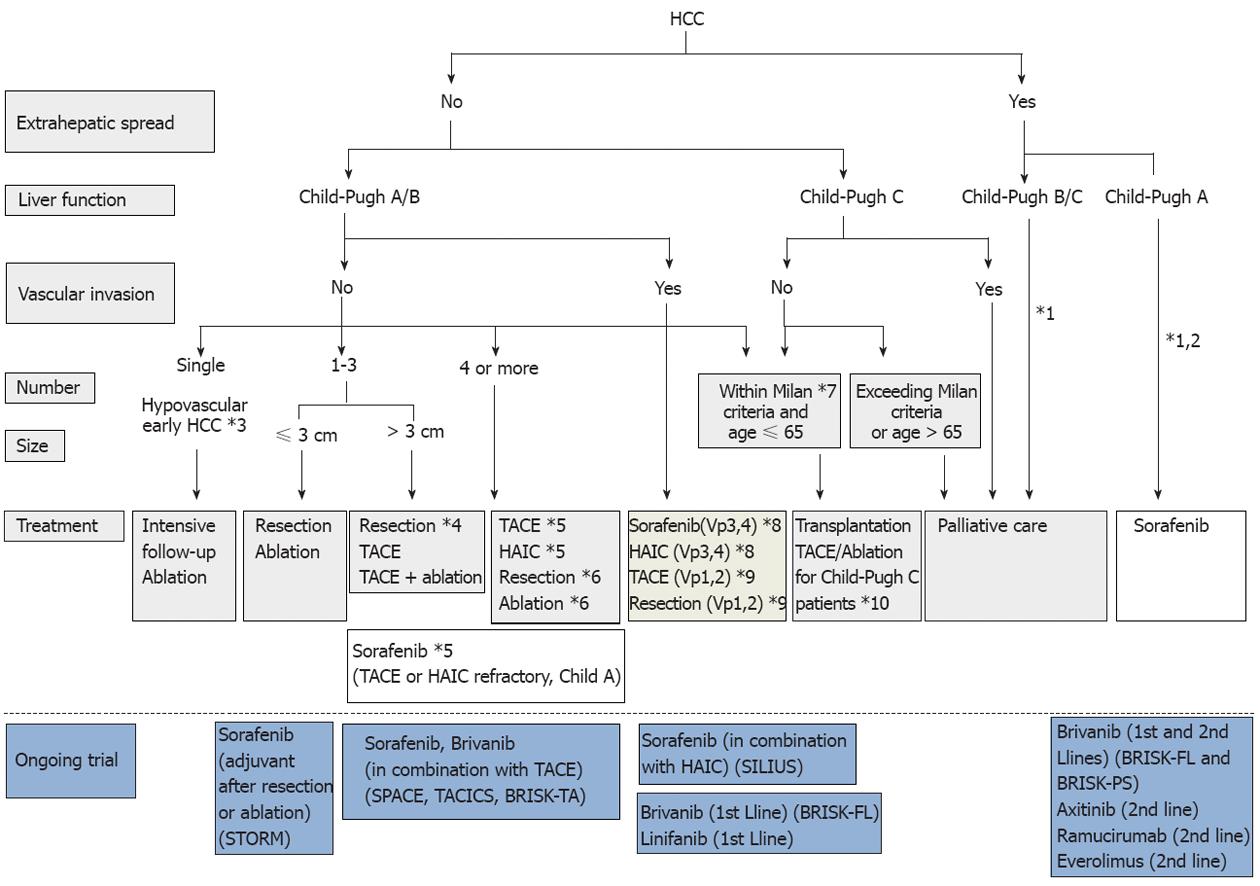
Figure 7 Consensus-based treatment algorithm for hepatocellular carcinoma proposed by Japan Society of Hepatology, revised in 2010.
(Cited and modified from Kudo et al[67] with permission.) *1: Treatment should be performed as if extrahepatic spread is negative, when extrahepatic spread is not considered as a prognostic factor in Child-Pugh class A/B patients; *2: Sorafenib is the first choice of treatment in this setting as a standard of care; *3: Intensive follow-up observation is recommended for hypovascular nodules by the Japanese Evidence-Based Clinical Practice Guidelines. However, local ablation therapy is frequently performed in the following cases: (1) when the nodule is diagnosed pathologically as early hepatocellular carcinoma (HCC); (2) when the nodules show decreased uptake on Gd-EOB-MRI, or (3) when the nodules show decreased portal flow by CTAP, since these nodules frequently progress to advanced HCC; *4: Even for HCC nodules exceeding 3 cm in diameter, transcatheter arterial chemoembolization (TACE) in combination with ablation is frequently performed when resection is not indicated; *5: TACE is the first choice of treatment in this setting. Hepatic arterial infusion chemotherapy (HAIC) using an implanted port is also recommended for TACE-refractory patients. The regimen for this treatment is usually low-dose FP [5-fluorouracil (5-FU) + CDDP] or intra-arterial 5-FU infusion combined with systemic interferon therapy. Sorafenib is also recommended for TACE or HAIC-refractory patients with Child-Pugh class A liver function; *6: Resection is sometimes performed when more than 4 nodules are detected. Ablation is sometimes performed in combination with TACE; *7: Milan criteria: Tumor size ≤ 3 cm and tumor number ≤ 3, or solitary tumor ≤ 5 cm. Even when liver function is good (Child-Pugh A/B), transplantation is sometimes considered for frequently recurring HCC patients; *8: Sorafenib and HAIC are recommended for HCC patients with major portal invasion such as Vp3 (portal invasion in the 1st portal branch) or Vp4 (portal invasion in the main portal branch); *9: Resection and TACE are frequently performed when portal invasion is minor, such as Vp1 (portal invasion in the 3rd or more peripheral portal branch) or Vp2 (portal invasion in the 2nd portal branch); *10: Local ablation therapy or subsegmental TACE is performed even for Child-Pugh C patients when transplantation is not indicated when there is no hepatic encephalopathy, no uncontrollable ascites, and a low bilirubin level (< 3.0 mg/dL). However, it is regarded as an experimental treatment because there is no evidence of a survival benefit in Child-Pugh C patients. A prospective study is necessary to clarify this issue.
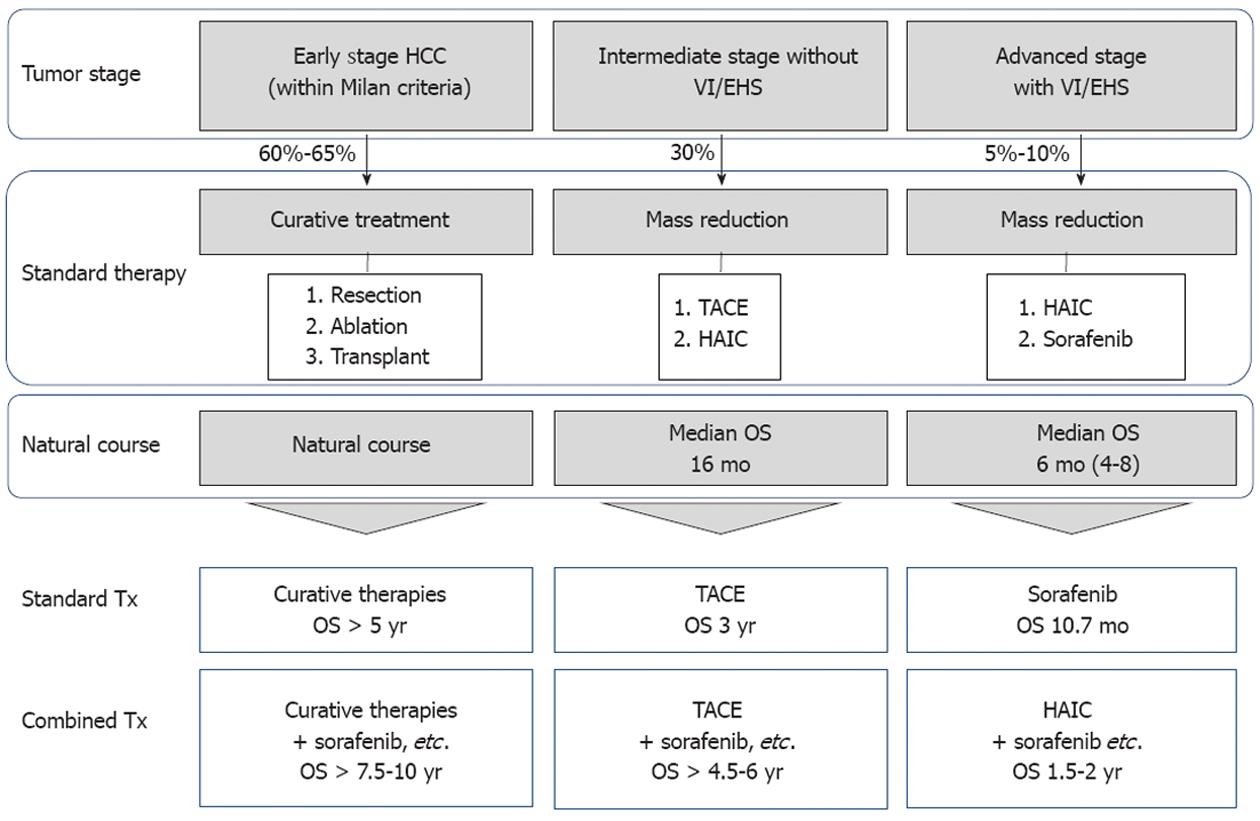
Figure 8 Outcomes of standard treatment modalities and expected future outcomes of combination therapy with molecular-targeted agents.
By combining molecular targeted agents with resection or ablation, life expectancy [overall survival (OS)] is expected to be prolonged to 7.5-10 years. In addition, for intermediate stage hepatocellular carcinoma (HCC), the prognosis is expected to be improved to 4.5-6 years by combination with transarterial chemoembolization (TACE). For advanced stage HCC, the prognosis is expected to be improved to 1.5-2 years by combination with hepatic arterial infusion chemotherapy (HAIC).
- Citation: Kudo M. Signaling pathway/molecular targets and new targeted agents under development in hepatocellular carcinoma. World J Gastroenterol 2012; 18(42): 6005-6017
- URL: https://www.wjgnet.com/1007-9327/full/v18/i42/6005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i42.6005