Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 7, 2011; 17(33): 3761-3775
Published online Sep 7, 2011. doi: 10.3748/wjg.v17.i33.3761
Published online Sep 7, 2011. doi: 10.3748/wjg.v17.i33.3761
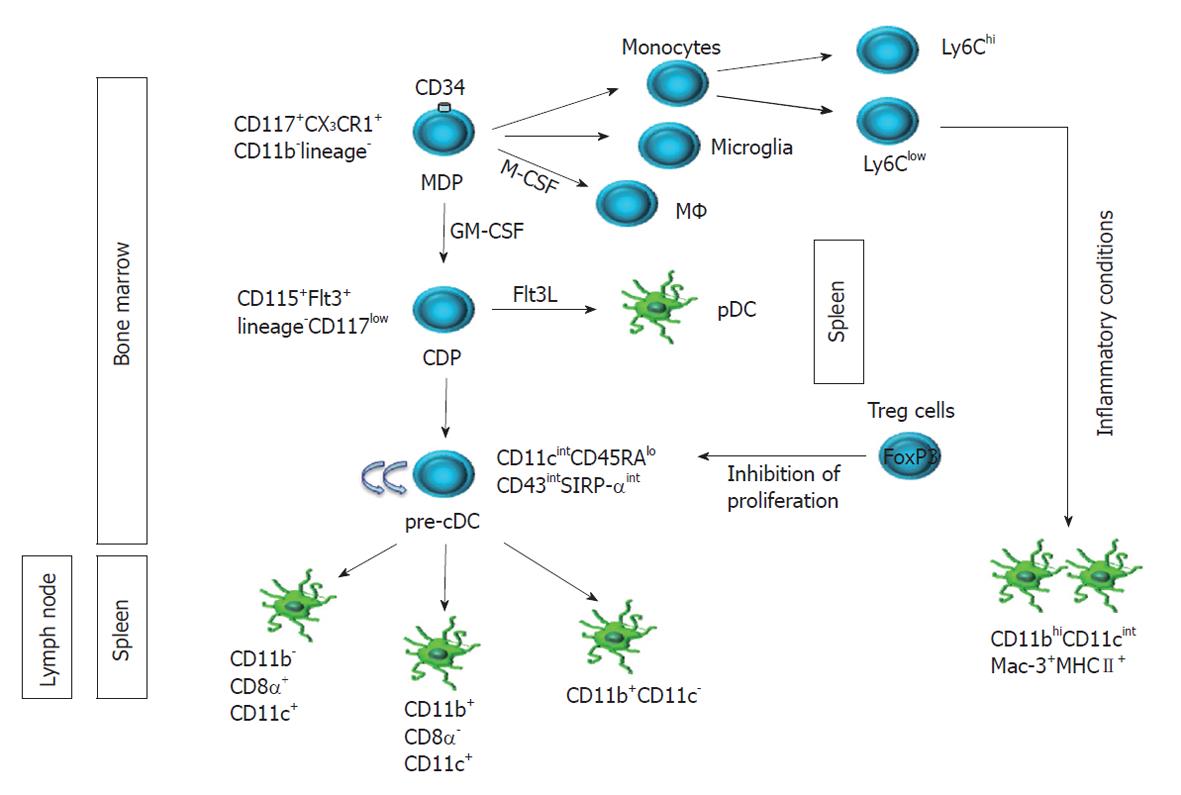
Figure 1 Ontogeny of dendritic cell subsets in mice.
The most recent evidence elucidating dendritic cell (DC) ontogeny and unraveling the complexity of the DC compartment in mice is summarized. The curved arrows in cyan denote proliferation potential. Regulatory T (Treg) cells may contribute to DC development and homeostasis in mice, as suggested by studies where Treg depletion has been associated with a 2- and 12-fold increase in precursor conventional DC (pre-cDC) and cDC in spleen and lymph node, respectively[14]. MDP: Macrophage and dendritic cell progenitor; CDP: Common dendritic cell progenitor; pDC: Plasmacytoid DC.
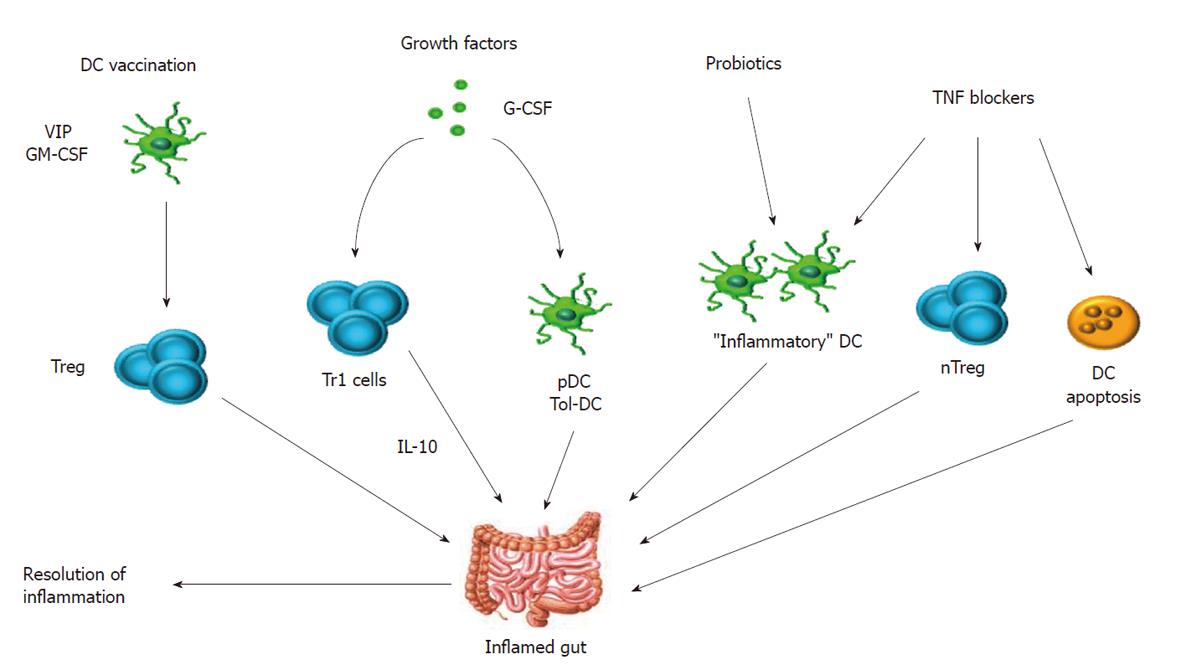
Figure 2 Potential strategies to modulate dendritic cell functionality in human inflammatory bowel disease.
In vitro differentiated tolerogenic dendritic cell (DC) have been administered to mice with inflammatory/autoimmune disorders. Vasoactive intestinal peptide (VIP) has a unique ability to skew DC function towards a tolerogenic profile and has been used to vaccinate animals with colitis, rheumatoid arthritis and post-transplantation graft-versus-host disease[76,77]. Selected growth factors have shown to modulate immune reactivity in vivo. For instance, granulocyte colony-stimulating factor (G-CSF) has been successfully given to patients with Crohn’s disease, leading to accumulation of pDC in the lamina propria and increase in IL-10 production, with favorable repercussions on disease manifestations[89]. GM-CSF: Granulocyte-macrophage colony-stimulating factor; IL: Interleukin; TNF: Tumor necrosis factor.
- Citation: Rutella S, Locatelli F. Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 2011; 17(33): 3761-3775
- URL: https://www.wjgnet.com/1007-9327/full/v17/i33/3761.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i33.3761