Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Aug 28, 2011; 17(32): 3691-3699
Published online Aug 28, 2011. doi: 10.3748/wjg.v17.i32.3691
Published online Aug 28, 2011. doi: 10.3748/wjg.v17.i32.3691
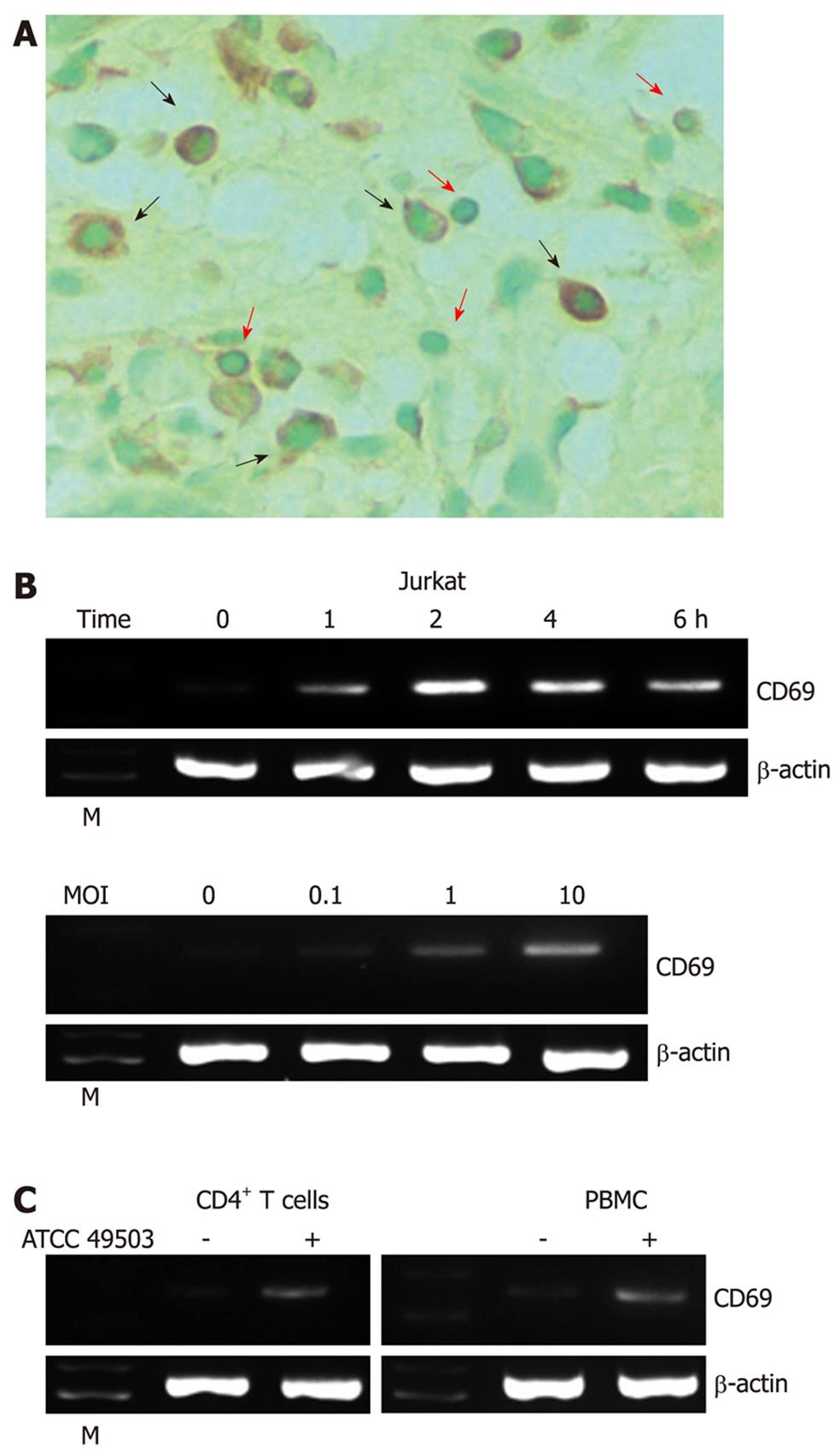
Figure 1 Expression of CD69 in Helicobacter pylori-infected T cells.
A: Immunohistochemical detection of CD69 in tissues of patients with Helicobacter pylori (H. pylori)-positive gastritis. Serial sections of gastric biopsy specimens were stained with a mouse monoclonal antibody to CD69 and counterstained with methyl green. Shown is a representative example of mucosa from a patient with H. pylori-positive gastritis. Note the positive staining for CD69 in lymphocytes as well as macrophages. Original magnification, × 800. The red and black arrows indicate the surfaces of lymphocytes and macrophages, respectively; B: H. pylori-induced CD69 mRNA expression in Jurkat cells. Total RNA was extracted from Jurkat cells infected with H. pylori strain ATCC 49503 [the multiplicity of infection (MOI) of 100] for the indicated time intervals and used for reverse transcription-polymerase chain reaction (RT-PCR) (top). Jurkat cells were infected with the indicated concentrations of ATCC 49503 for 2 h. Total RNA was extracted and used for RT-PCR (bottom); C: H. pylori-induced CD69 mRNA expression in peripheral blood mononuclear cells (PBMCs) and CD4+ T cells. Total RNA was extracted from PBMCs and CD4+ T cells infected with ATCC 49503 for 2 h and used for RT-PCR (MOI of 10). β-actin expression served as a control. Lane M: Markers.
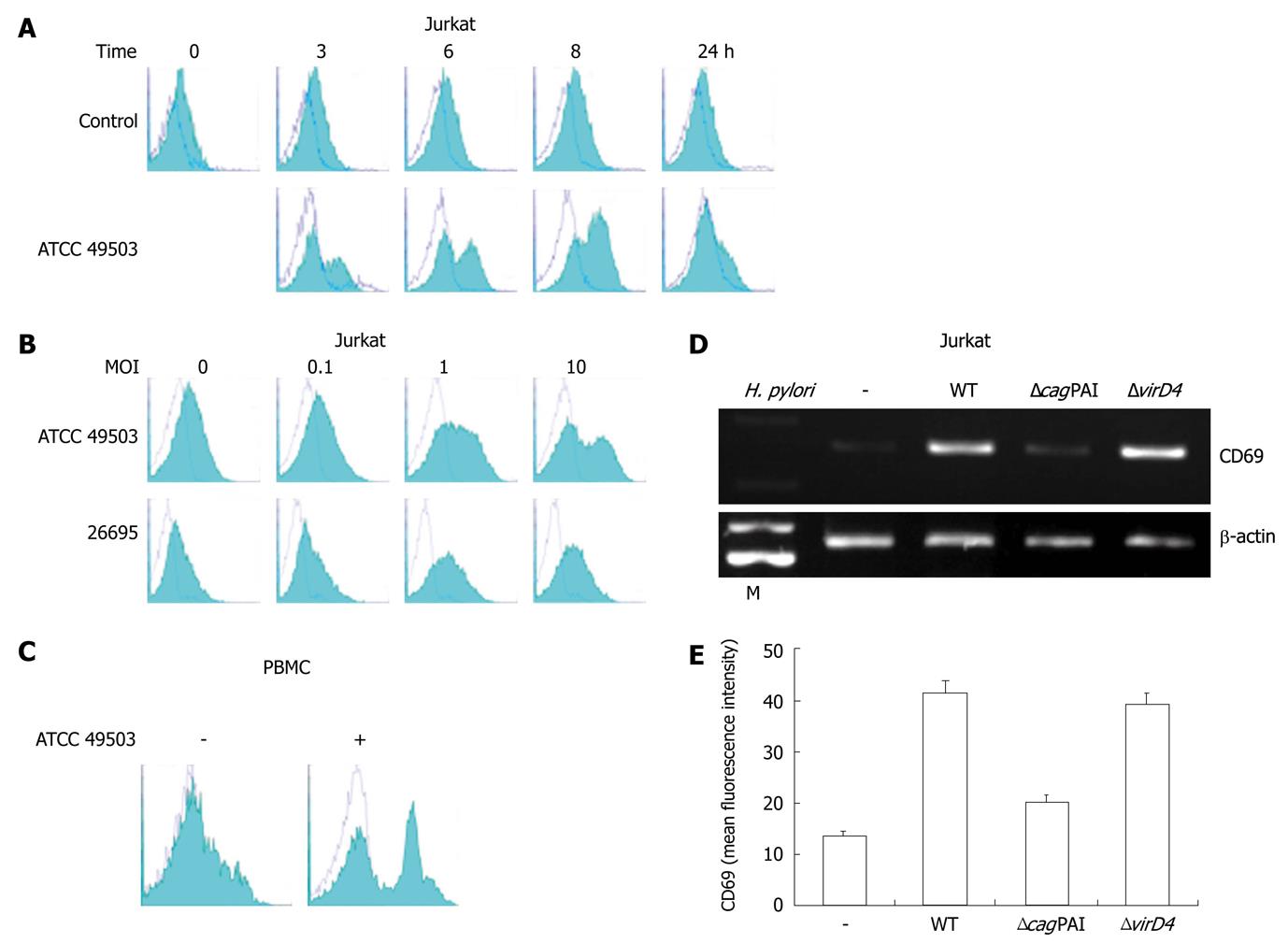
Figure 2 CD69 expression on Jurkat cells.
A: Time course of cell surface expression of CD69 on Jurkat cells exposed to Helicobacter pylori (H. pylori). Jurkat cells were cultured for the indicated times in culture medium (control) or in the presence of ATCC 49503 [the multiplicity of infection (MOI) of 10]. After cell harvest, CD69 expression on the cells was determined by flow cytometry; B: H. pylori infection increases cell surface expression of CD69 on Jurkat cells in a dose-dependent fashion. Jurkat cells were infected with different concentrations of H. pylori strains, ATCC 49503 and 26695, and CD69 levels were measured by flow cytometry on cells harvested after 8 h; C: H. pylori infection increases cell surface expression of CD69 on peripheral blood mononuclear cells (PBMCs). PBMCs were infected with ATCC 49503 (MOI of 10), and CD69 levels were measured on cells harvested after 8 h; D: cag pathogenicity island (cagPAI) products of H. pylori are required for the induction of CD69 mRNA expression. Total RNA was extracted from Jurkat cells that had been infected with the wild-type strain 26695 (WT) or the isogenic mutants ΔcagPAI and ΔvirD4 (MOI of 10) for 2 h and used for reverse transcription-polymerase chain reaction. Lane M: Markers; E: Flow cytometric analysis was carried out for the surface expression of CD69 in Jurkat cells infected with the wild-type strain 26695 (WT) or the isogenic mutants ΔcagPAI and ΔvirD4. Jurkat cells were infected for 8 h with various H. pylori strains (MOI of 10). Cells were stained with phycoerythrin-labeled monoclonal antibody. Datas are mean ± SD of three experiments.
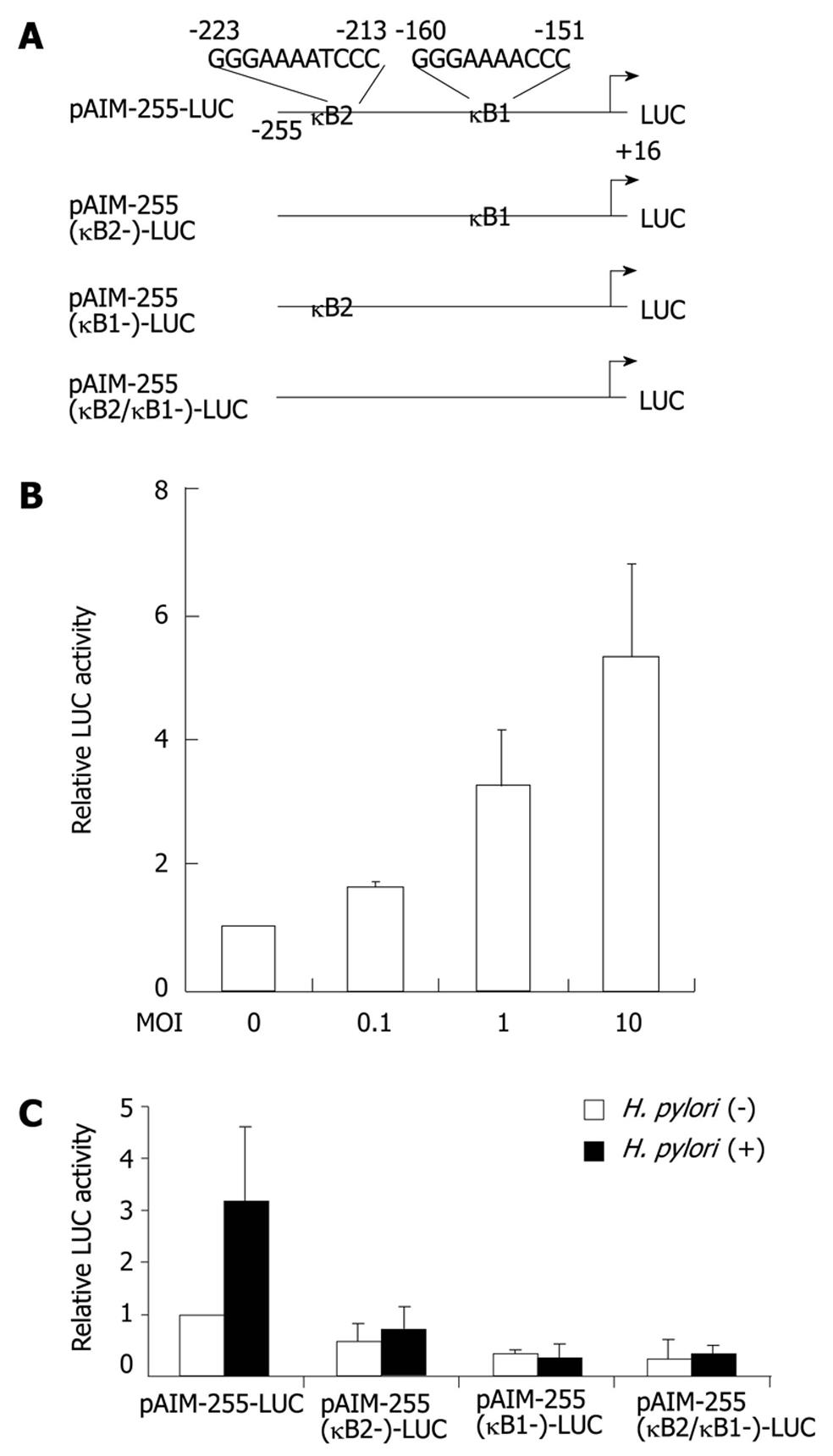
Figure 3 Helicobacter pylori activates the CD69 promoter through two nuclear factor-κB binding sites.
A: Schematic diagram of the CD69 reporter constructs containing the wild-type (pAIM-255-LUC) and internal deletion mutants of κB1 and/or κB2 motifs. LUC: Luciferase; B: Helicobacter pylori (H. pylori) infection increases CD69 promoter activity in a dose-dependent fashion. pAIM-255-LUC was transfected into Jurkat cells, and the cells were subsequently infected with H. pylori ATCC 49503 for 6 h; C: The indicated CD69 reporter constructs were transfected into Jurkat cells, and subsequently the cells were infected with ATCC 49503 for 6 h (the multiplicity of infection of 10). The activity is expressed relative to that of cells transfected with pAIM-255-LUC without further H. pylori infection, which was defined as 1. Datas are mean ± SD of three experiments.
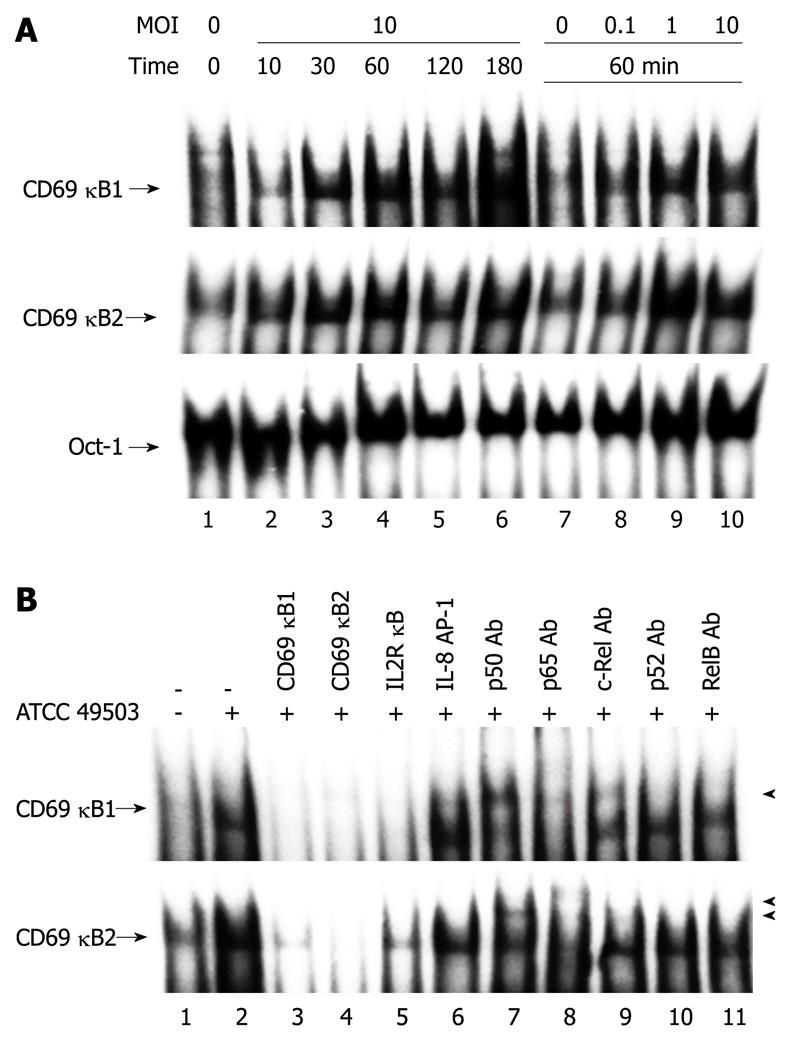
Figure 4 Helicobacter pylori infection induces nuclear factor-κB binding activity.
A: Nuclear factor (NF)-κB activation in Jurkat cells infected with Helicobacter pylori (H. pylori), as evaluated by electrophoretic mobility shift assay (Oct-1). Nuclear extracts from Jurkat cells infected with different densities [the multiplicity of infection (MOI)] of H. pylori ATCC 49503 (lanes 7 to 10) for the indicated times (lanes 1 to 6) were mixed with oligonucleotide probes CD69 κB1 (top) and CD69 κB2 (middle), which contained the putative NF-κB motifs located at positions -160 and -223, respectively; B: Competition assays were performed with nuclear extracts from Jurkat cells infected with ATCC 49503 (MOI of 10) for 180 min. Where indicated, the excess amounts of each specific competitor oligonucleotide were added to the reaction mixture with each oligonucleotide probe (lanes 3 to 6). A supershift assay of NF-κB DNA binding complexes in the same nuclear extracts was also performed. Where indicated, appropriate antibodies (Ab) were added to the reaction mixture before the addition of probe CD69 κB1 (top) or CD69 κB2 (bottom). Arrows indicate the specific complexes, while arrowheads indicate the DNA binding complexes supershifted by the antibodies.
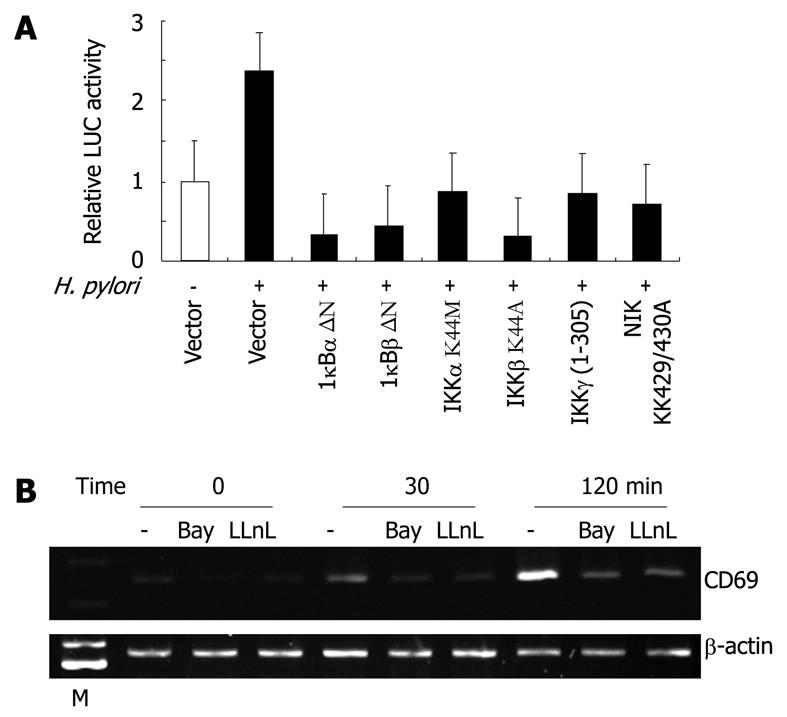
Figure 5 Nuclear factor-κB signal is essential for the activation of CD69 expression by Helicobacter pylori in T cells.
A: Functional effects of IκBα-, IκBβ-, and IKKγ-dominant-interfering mutants and kinase-deficient IKKα, IKKβ, and NIK mutants on Helicobacter pylori (H. pylori)-induced activation of the CD69 promoter. Jurkat cells were transfected with the CD69 reporter construct (pAIM-255-LUC) and the indicated mutant plasmids or empty vector (pCMV4) and then infected with H. pylori ATCC 49503 for 6 h. Open bar: Luciferase (LUC) activity of the CD69 reporter construct and pCMV4 without H. pylori infection. All values were calculated as the change (n-fold) in induction values relative to the basal level measured in uninfected cells. Data are mean ± SD of three independent experiments. B: Bay 11-7082 and N-acetyl-L-leucyl-L-leucyl-L-norleucinal (LLnL) inhibit CD69 mRNA expression induced by H. pylori. Jurkat cells were pretreated with Bay 11-7082 (20 μmol/L) or LLnL (20 μmol/L) for 2 h prior to H. pylori infection and subsequently infected with H. pylori ATCC 49503 for 30 or 120 min. CD69 mRNA expression on harvested cells was analyzed by reverse transcription-polymerase chain reaction. Lane M: Markers.
-
Citation: Mori N, Ishikawa C, Senba M. Induction of CD69 expression by
cag PAI-positiveHelicobacter pylori infection. World J Gastroenterol 2011; 17(32): 3691-3699 - URL: https://www.wjgnet.com/1007-9327/full/v17/i32/3691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i32.3691