Copyright
©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 28, 2010; 16(44): 5582-5587
Published online Nov 28, 2010. doi: 10.3748/wjg.v16.i44.5582
Published online Nov 28, 2010. doi: 10.3748/wjg.v16.i44.5582
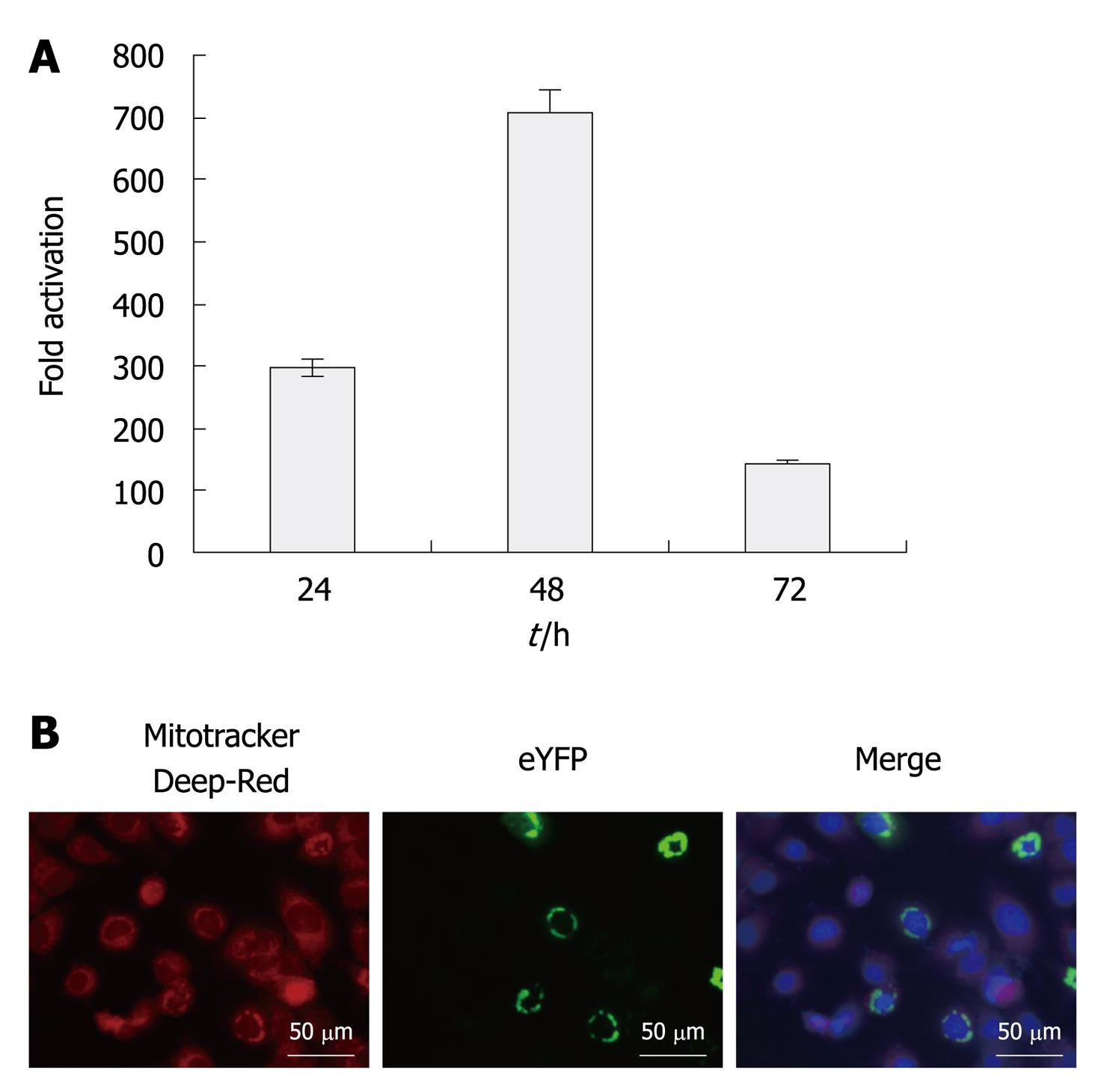
Figure 1 Activation of the interferon-β promoter by enhanced yellow fluorescent protein-mitochondrial antiviral signaling protein and subcellular localization of enhanced yellow fluorescent protein-mitochondrial antiviral signaling protein.
A: Activation of the interferon (IFN)-β promoter by enhanced yellow fluorescent protein (eYFP)-mitochondrial antiviral signaling protein (MAVS). Expression vector of eYFP-MAVS was co-transfected with IFN-β-secreted placental alkaline phosphatase (SEAP) in Huh7.5 cells. pRL-TK was co-transfected to normalize transfection efficiency. SEAP activity in cell culture was measured at 24, 48 and 72 h post-transfection. Results are expressed as activation levels of the promoter compared to those in cells transfected with an empty expression vector. The error bars represent the SDs from the mean values obtained from three independent experiments performed in duplicate; B: Fluorescence microscopy of Huh7.5 cells transfected with eYFP-MAVS at 48 h post-transfection. Mitochondria were stained with Mitotracker deep red (red) and nuclei were labeled with 4’,6-Diamidino-2-phenylindole (blue). Yellow labeling in the merged image indicates co-localization of eYFP-MAVS with mitochondria.
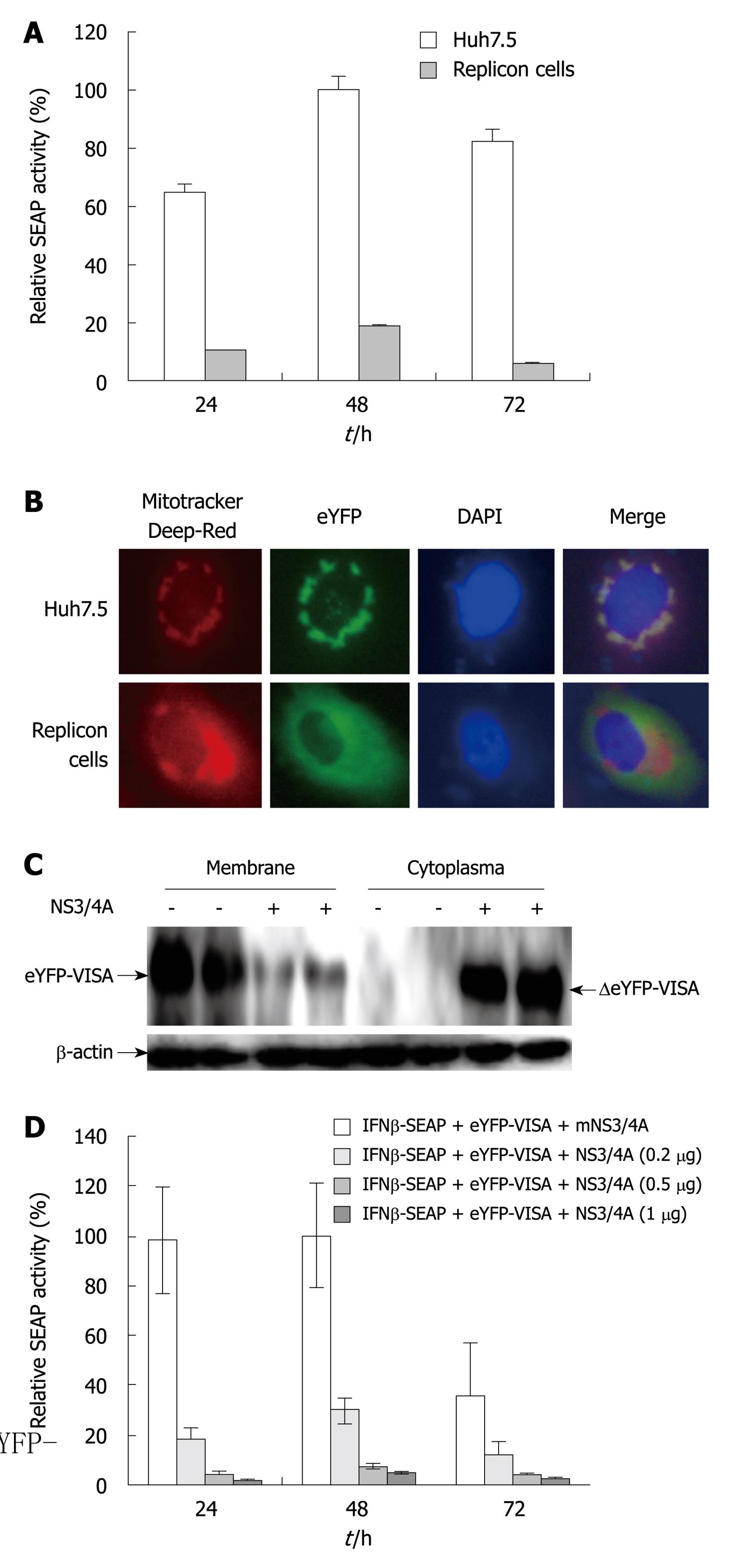
Figure 2 Hepatitis C virus NS3/4A protease activity impairs the enhanced yellow fluorescent protein-mitochondrial antiviral signaling protein/interferon-β-secreted placental alkaline phosphatase signaling pathway.
A: Validation of the reporter assay system in Huh7.5 cells that contain full-length hepatitis C virus (HCV) replicons (P < 0.05). Huh7.5 and replicon cells were co-transfected with enhanced yellow fluorescent protein (eYFP)-mitochondrial antiviral signaling protein (MAVS) and interferon (IFN)-β-secreted placental alkaline phosphatase (SEAP). pRL-TK was co-transfected to normalize transfection efficiency. SEAP activity was examined at 24, 48 and 72 h after transfection. Bars indicate SD (n = 3); B: Localization of eYFP-MAVS. Subcellular localization of eYFP-MAVS was assessed by fluorescence microscopy 48 h post-transfection in Huh7.5 and replicon cells; C: Western blotting analysis of eYFP-MAVS cleaved by NS3/4A protease. Lysates of Huh7.5 and replicon cells treated as above were harvested at 48 h post-transfection and analyzed by Western blotting. Arrows indicate the positions of eYFP-MAVS and ΔeYFP-MAVS, respectively; D: Huh7.5 cells were co-transfected with eYFP-MAVS, IFN-β-SEAP and increasing amounts of expression plasmid pNS3/4A that encoded HCV NS3/4A protease (0, 0.2, 0.5 and 1 μg). pRL-TK was co-transfected to normalize transfection efficiency. SEAP activity in cell culture was measured at 24, 48 and 72 h post-transfection. Bars indicate SD (n = 3).
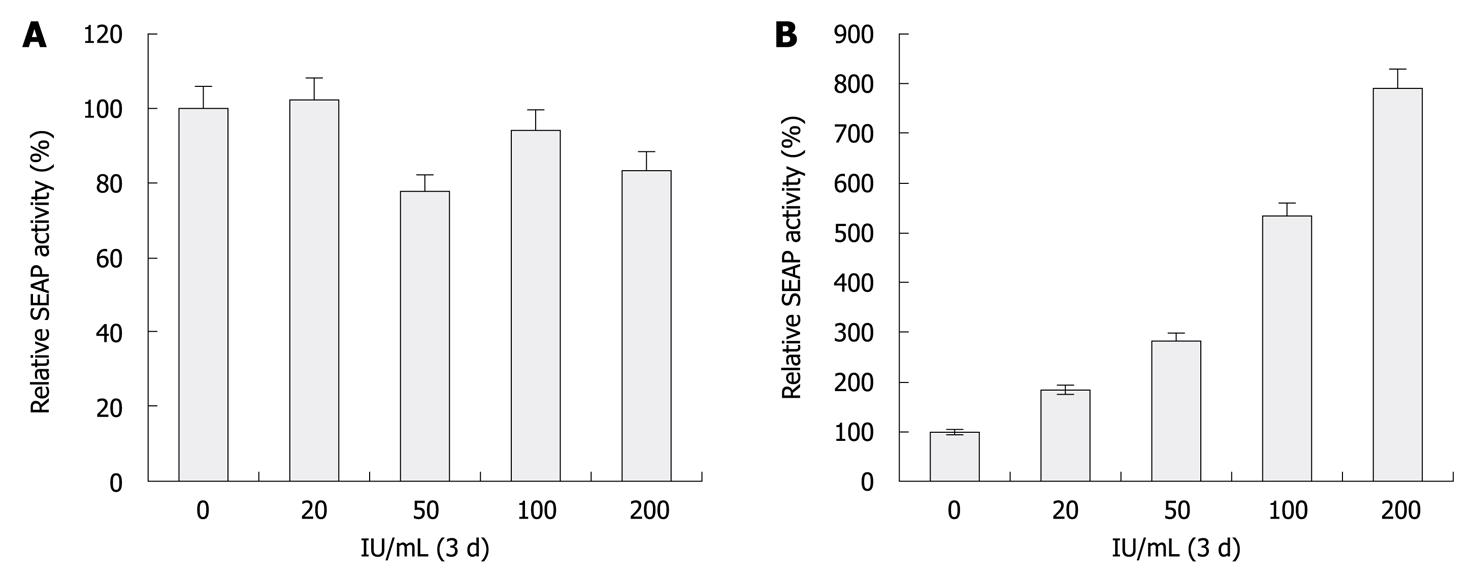
Figure 3 Secreted placental alkaline phosphatase reporter correlates with the anti-hepatitis C virus activity of interferon-α.
A, B: Increase of secreted placental alkaline phosphatase (SEAP) activity in enhanced yellow fluorescent protein (eYFP)-mitochondrial antiviral signaling protein (MAVS) and interferon (IFN)-β-SEAP co-transfected Huh7.5 (P > 0.05) and replicon cells (P < 0.05) treated with IFN-α. Before transfection, Huh7.5 and replicon cells were incubated in the absence or presence of 20, 50, 100 and 200 IU/mL IFN-α for 72 h. SEAP activity was measured at 48 h post-transfection in the presence of IFN-α. The percentage increase in luciferase activity relative to the untreated controls was plotted. Bars indicate SD (n = 3).
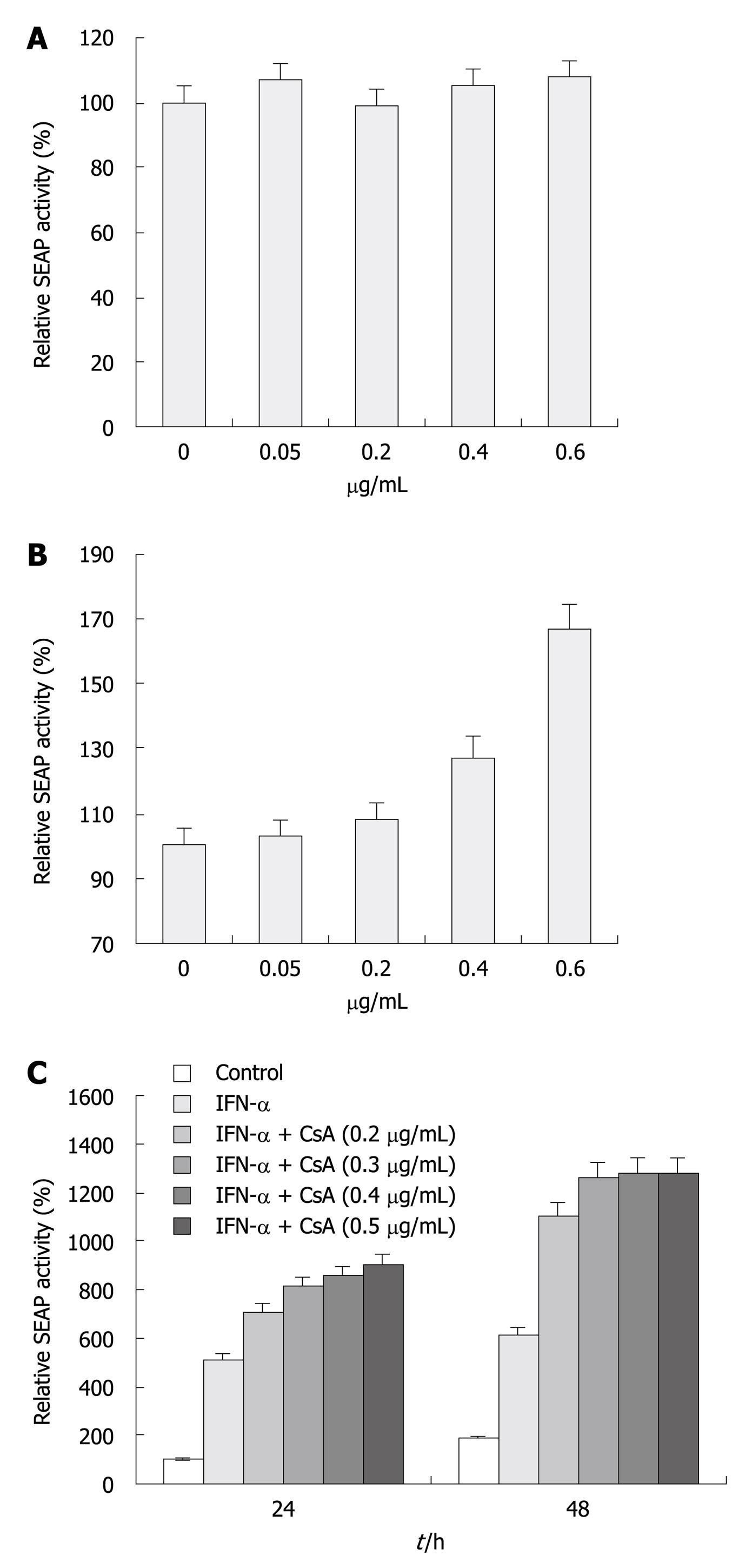
Figure 4 Inhibitory effect of cyclosporine A on hepatitis C virus replication and its combination effect with interferon-α.
A, B: Increase of secreted placental alkaline phosphatase (SEAP) activity in enhanced yellow fluorescent protein (eYFP)-mitochondrial antiviral signaling protein (MAVS) and interferon (IFN)-β-SEAP co-transfected Huh7.5 (P > 0.05) and replicon (P < 0.05) cells treated with cyclosporine A (CsA). Before transfection, Huh7.5 and replicon cells were incubated with CsA at 0, 0.05, 0.2, 0.4 and 0.6 μg/mL for 48 h. SEAP activity was measured at 24 h post-transfection in the presence of CsA. The percentage increase in SEAP activity relative to the untreated controls was plotted. Bars indicate SD (n = 3); C: IFN-α in combination with CsA therapy enhanced its inhibitory effect. Replicon cells were incubated with IFN-α (100 IU/mL) in combination with CsA at 0, 0.2, 0.3, 0.4 and 0.5 μg/mL for 48 h, and co-transfected with eYFP-MAVS and IFN-β-SEAP; pRL-TK was co-transfected to normalize transfection efficiency. SEAP activity was measured at 24 and 48 h post-transfection in the presence of IFN-α and CsA. The percentage increase of SEAP activity relative to the untreated controls was plotted. Bars indicate SD (n = 3).
- Citation: Fu QX, Wang LC, Jia SZ, Gao B, Zhou Y, Du J, Wang YL, Wang XH, Peng JC, Zhan LS. Screening compounds against HCV based on MAVS/IFN-β pathway in a replicon model. World J Gastroenterol 2010; 16(44): 5582-5587
- URL: https://www.wjgnet.com/1007-9327/full/v16/i44/5582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i44.5582