Copyright
©2010 Baishideng.
World J Gastroenterol. Apr 14, 2010; 16(14): 1747-1752
Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1747
Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1747
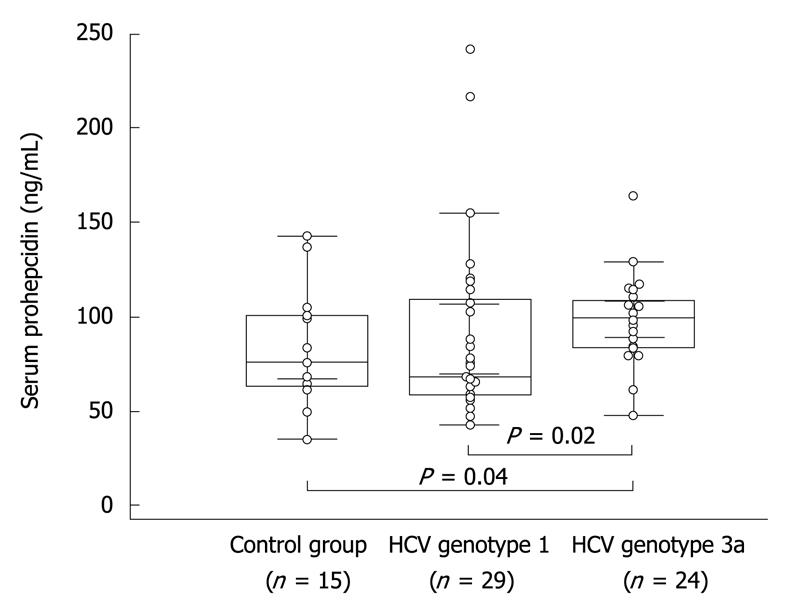
Figure 1 Baseline concentration of prohepcidin in healthy individuals as well as in hepatitis C virus (HCV) genotype 1 and genotype 3 patients.
Dots indicate individual values in studied groups, boxes depict mean and standard error of mean, bars show standard deviations.
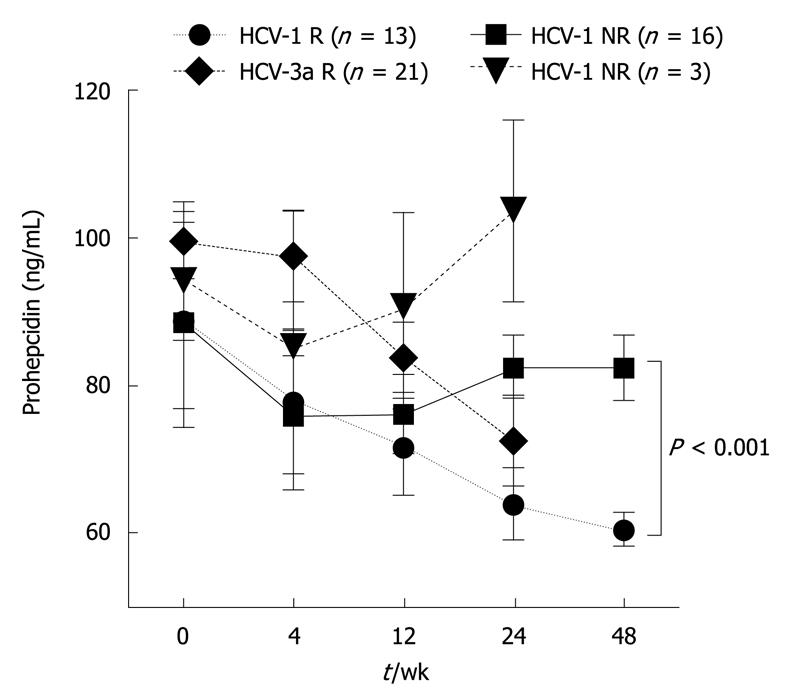
Figure 2 Serum prohepcidin concentrations in chronic hepatitis C during antiviral therapy with respect to the treatment response.
R: Responders [HCV-RNA undetectable 6 mo after the end of pegylated interferon (PEG-IFN) + ribavirin (RBV) therapy]; NR: Non-responders (HCV-RNA positive 6 mo after the end of PEG-IFN + RBV therapy).
- Citation: Jaroszewicz J, Rogalska M, Flisiak I, Flisiak R. Successful antiviral therapy is associated with a decrease of serum prohepcidin in chronic hepatitis C. World J Gastroenterol 2010; 16(14): 1747-1752
- URL: https://www.wjgnet.com/1007-9327/full/v16/i14/1747.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i14.1747