Copyright
©2009 The WJG Press and Baishideng.
World J Gastroenterol. Nov 7, 2009; 15(41): 5129-5140
Published online Nov 7, 2009. doi: 10.3748/wjg.15.5129
Published online Nov 7, 2009. doi: 10.3748/wjg.15.5129
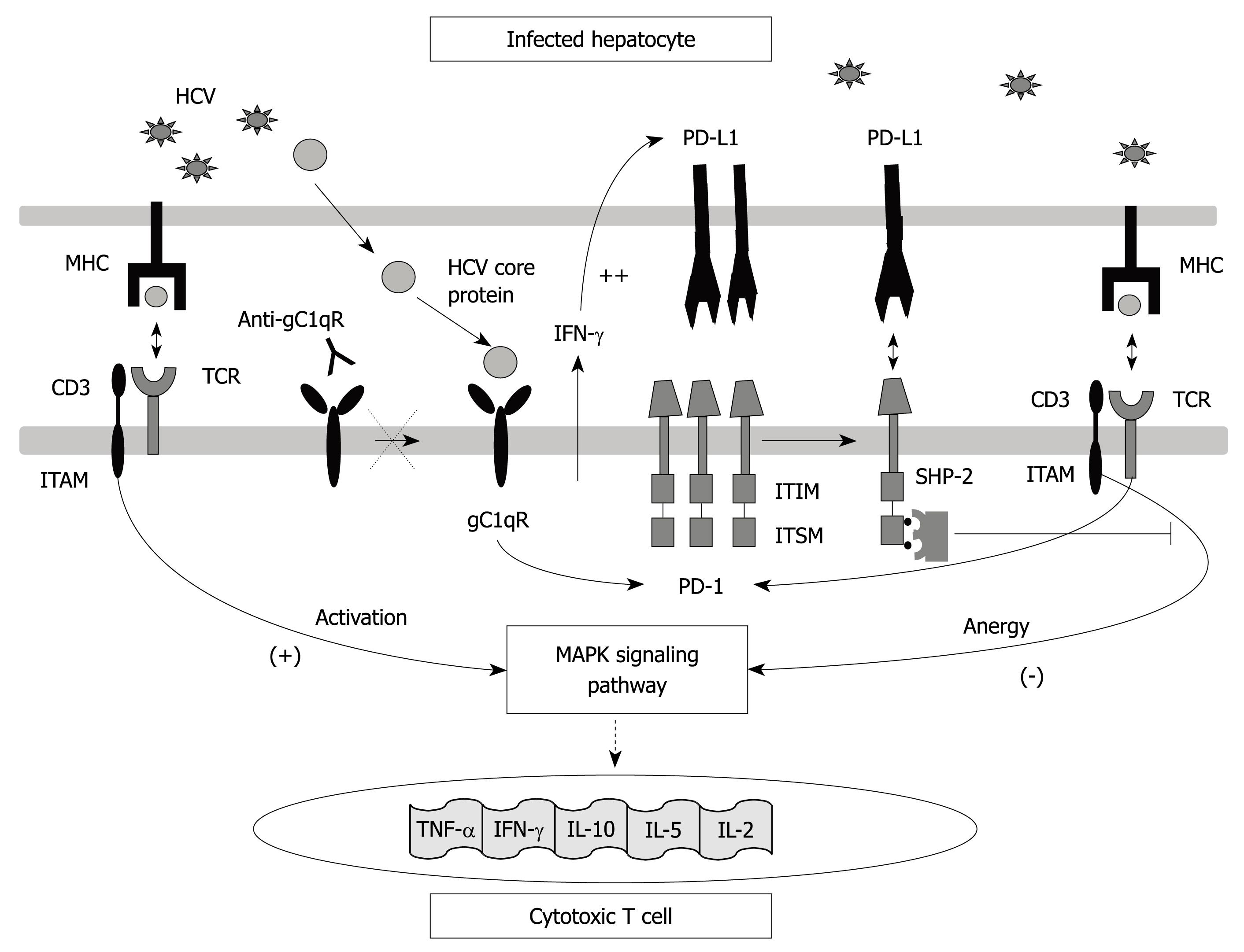
Figure 1 Programmed death-1 (PD-1) structure and interactions.
Interaction between the PD-1 molecule expressed on T cells and its ligand PD-L1 expressed on antigen-presenting cells leads to immunoreceptor tyrosine-based switch motif (ITSM) motif phosphorylation in its cytoplasmic tyrosines which are recognized by src homology 2 domain-containing tyrosine phosphatase 2 (SHP-2). All of these interactions cause T cell anergy due to T cell receptor (TCR)-dependent MAP Kinase-pathway signalling inhibition which avoids interleukin (IL)-2 gene transduction. PD-1 expression is induced by TCR activation but could also be favoured by HCV-core protein through interaction with gC1qR. PD-L1 is up-regulated on antigen presenting cells by the effect of γ-interferon produced during HCV infection by activated lymphocytes.
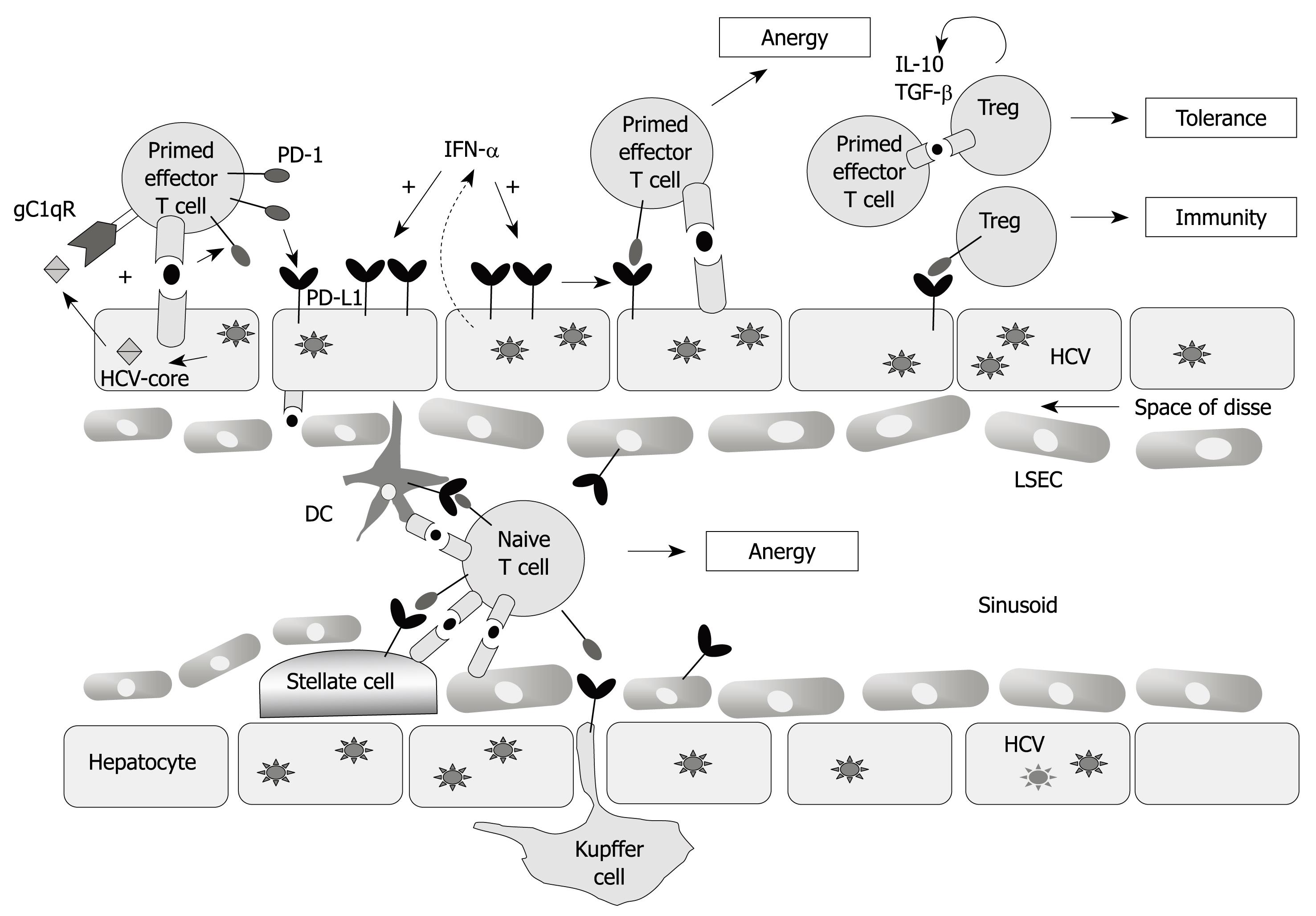
Figure 2 Liver microenvironment.
Circulating HCV-specific CD8+ T cells migrating through the hepatic sinusoid interact with resident liver cells [Kupffer cells, dendritic cells (DC), hepatocytes, liver sinusoidal endothelial cells (LSECs), stellate cells] that could act as antigen presenting cells. These cells up-regulate PD-L1 expression during persistent HCV infection and interact with the PD-1 molecule expressed on HCV-specific CD8+ T cells. This interaction leads to T cell anergy. PD-1 up-regulation is produced by TCR stimulation in addition to the interaction between HCV-core protein and the complement receptor (gC1qR). In this micro-environment the regulatory T cells (Treg) also participate, whose activity is also regulated by the PD-1/PD-L1 pathway.
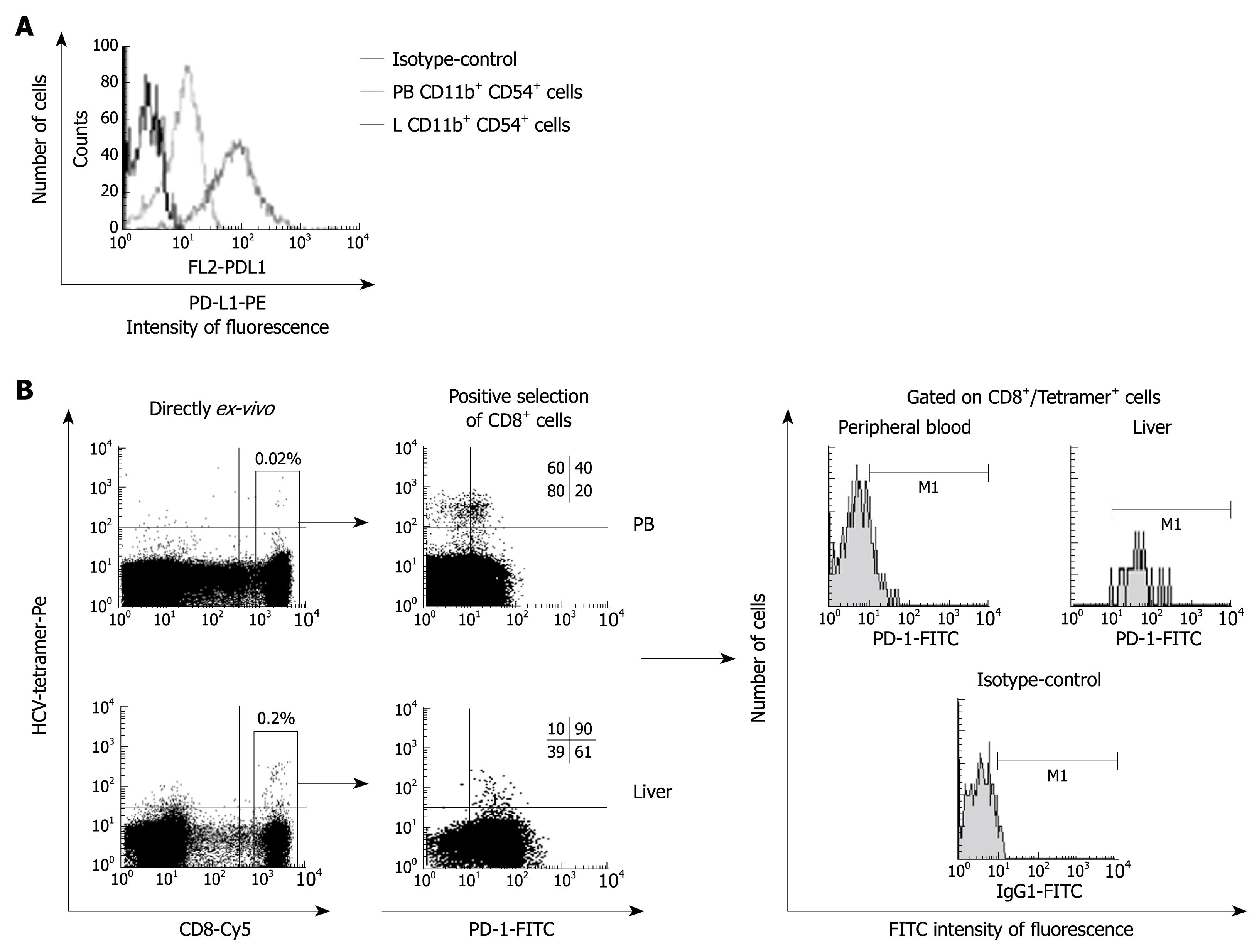
Figure 3 PD-L1 expression on Kupffer cells and PD-1 expression on HCV-specific CD8+ T cells.
A: PD-L1-FITC FACS® histograms gated on CD11b+ CD54+ cells from liver (L) and peripheral blood (PB), showing a higher PD-L1 expression on intrahepatic Kuppfer cells; B: FACS® dot-plots and histograms of peripheral blood and intrahepatic T cells stained with CD8-Cy mAb, HCV-tetramers-PE and PD-1-FITC mAb. Gated Tetramer+/CD8+ cells are presented in the histograms for PD-1-FITC expression. PD-1 is up-regulated on intrahepatic HCV-specific CD8+ cells.
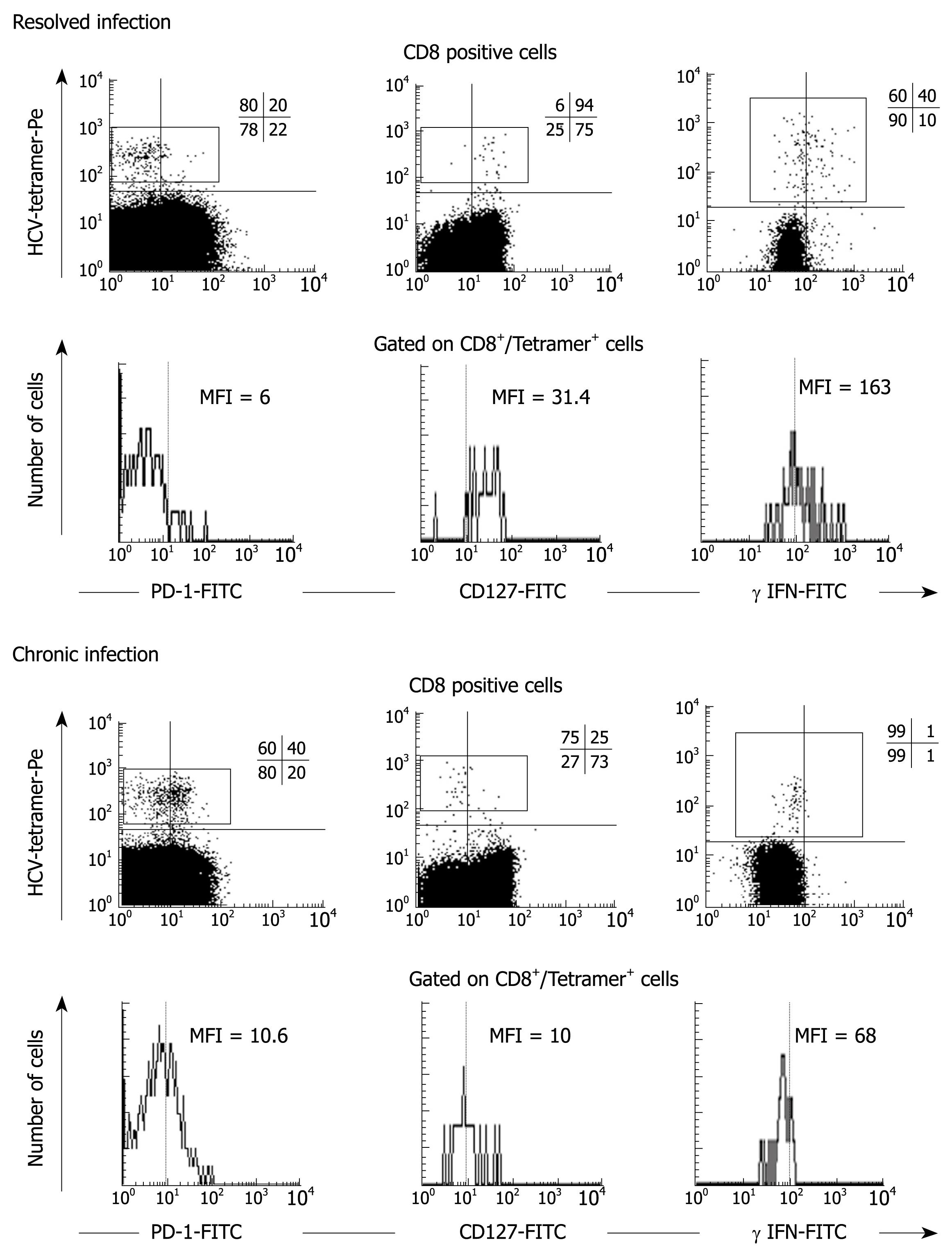
Figure 4 Direct ex-vivo PD-1, CD127 expression and IFN-γ production.
FACS® dot-plots and histograms of peripheral blood T cells from two representative patients, one with persistent HCV infection and the other with resolved HCV infection. T cells are stained with PD-1-FITC, CD127-FITC, γ-IFN-FITC and CD8-Cy mAbs and HCV-tetramers. In persistent infection, HCV-specific CD8+ cells maintain a PD-1+/CD127-/IFN-γ- phenotype while the resolved infection case displays an opposite phenotype PD-1-/CD127+/IFN-γ+.
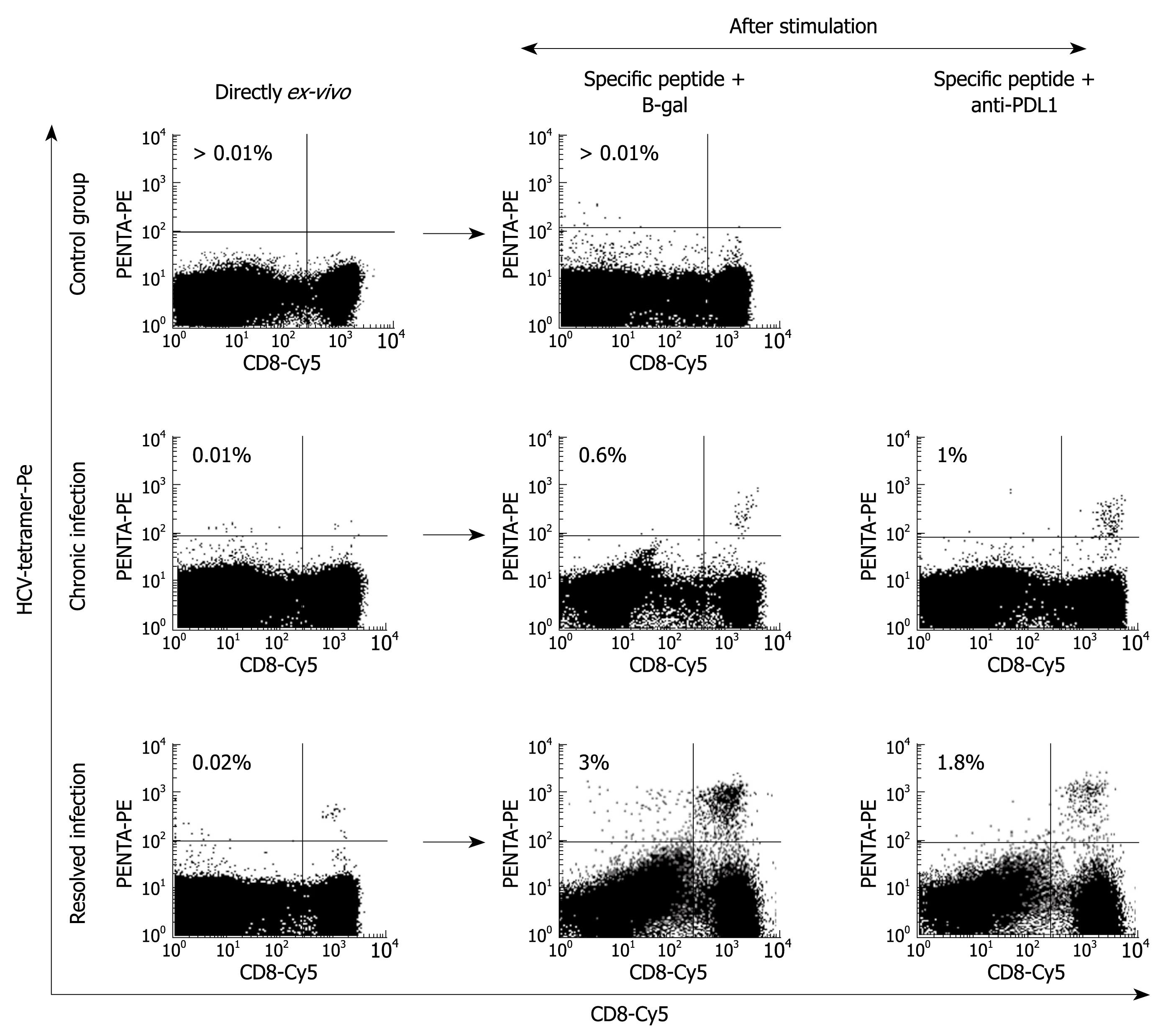
Figure 5 Proliferation restoration after PD-1 blockade.
FACS® dot-plots of peripheral blood T cells from two representative patients, one with persistent HCV infection and the other with resolved HCV infection, and a control case, after specific stimulation in the presence or absence of anti-PD-L1 mAb. T cells were stimulated for 10 d with the HCV–specific peptide plus IL-2. After stimulation, T cells were stained with CD8-Cy mAbs and HCV-tetramers-PE. PD-1/PD-L1 pathway blockade by anti-PD-L1 antibodies increases the HCV-specific cell proliferation in the chronic patient that had a high level of PD-1 expression.
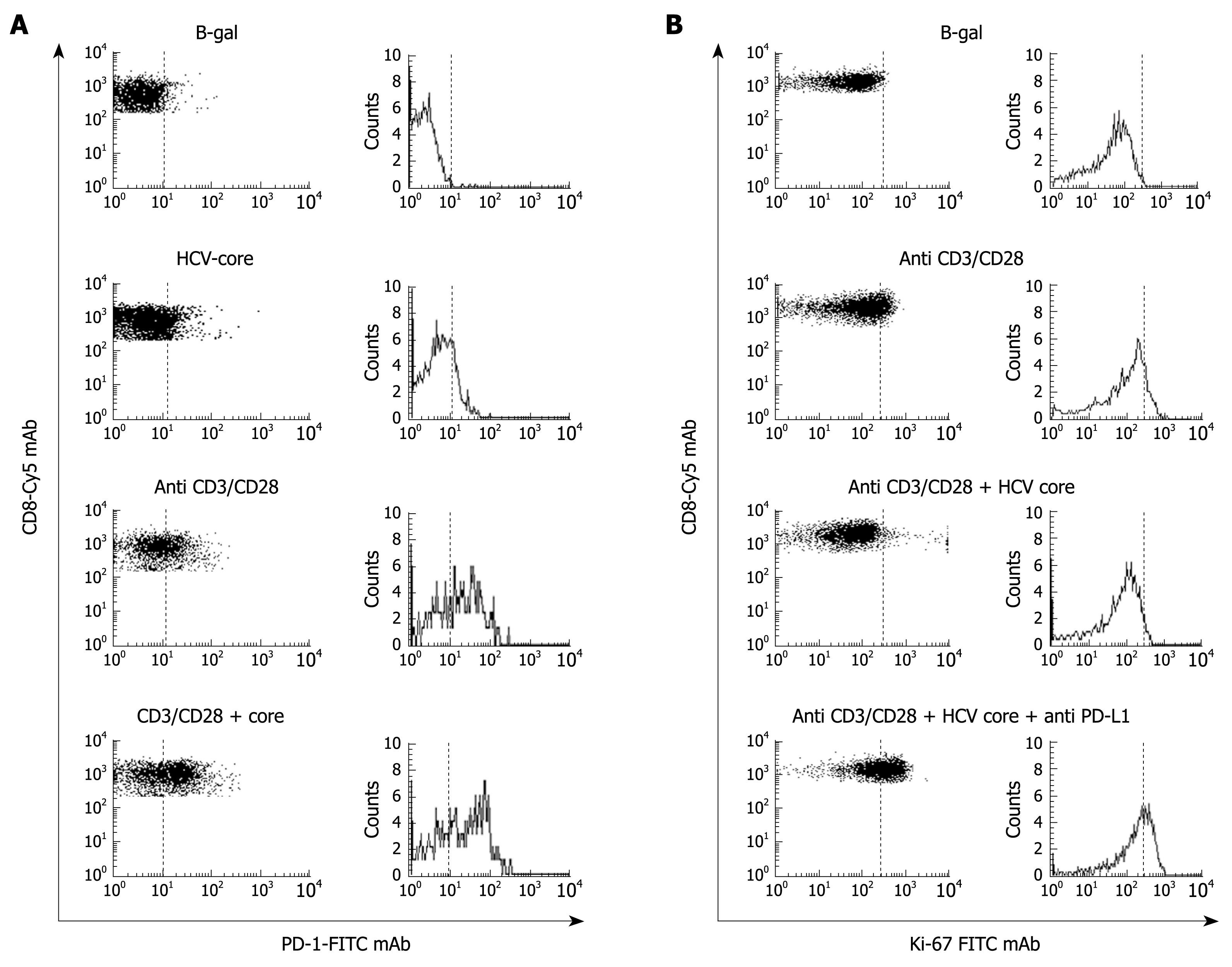
Figure 6 PD-1 up-regulation induced by HCV-core protein.
A: FACS® dot-plots and histograms of peripheral blood CD8+ cells stained with PD-1-FITC and CD8-Cy mAbs from a healthy subject. CD8+ cells were stimulated with B-galactosidase, HCV-core protein, CD3-CD28 mAb, and HCV-core protein plus CD3-CD28 mAbs. PD-1 expression was highly up-regulated on CD8+ cells after non-specific stimulation by anti CD3/CD28 mAbs in the presence of HCV core protein; B: FACS® dot-plots and histograms of peripheral blood CD8+ cells stained with Ki-67 FTIC and CD8-Cy mAbs from the same healthy subject to test proliferation ability of CD8+ cells after incubation with B-galactosidase, CD3-CD28 mAb, HCV-core protein plus CD3-CD28 mAb and HCV-core protein plus CD3-CD28 and anti-PD-L1 mAbs. HCV-core protein decreased the proliferation induced by CD3-CD28 mAb stimulation. This proliferation impairment induced by HCV-core protein was resolved by anti-PD-L1 mAb treatment.
- Citation: Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C. World J Gastroenterol 2009; 15(41): 5129-5140
- URL: https://www.wjgnet.com/1007-9327/full/v15/i41/5129.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5129