Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Aug 21, 2007; 13(31): 4230-4235
Published online Aug 21, 2007. doi: 10.3748/wjg.v13.i31.4230
Published online Aug 21, 2007. doi: 10.3748/wjg.v13.i31.4230
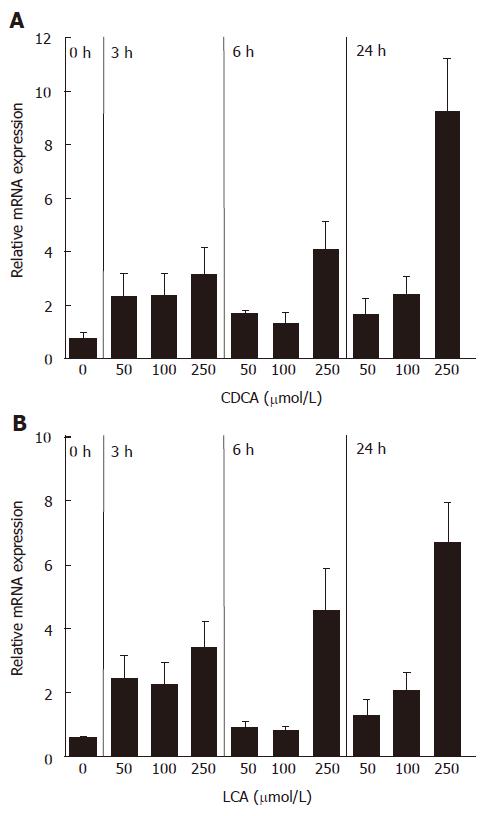
Figure 1 mRNA induction of FGF19 in LS174T cells by CDCA (A) and LCA (B) (mean ± SD, Student’s t-test).
P < 0.05 between 3 h, 6 h and 24 h treatment groups and 0 h group in both A and B.
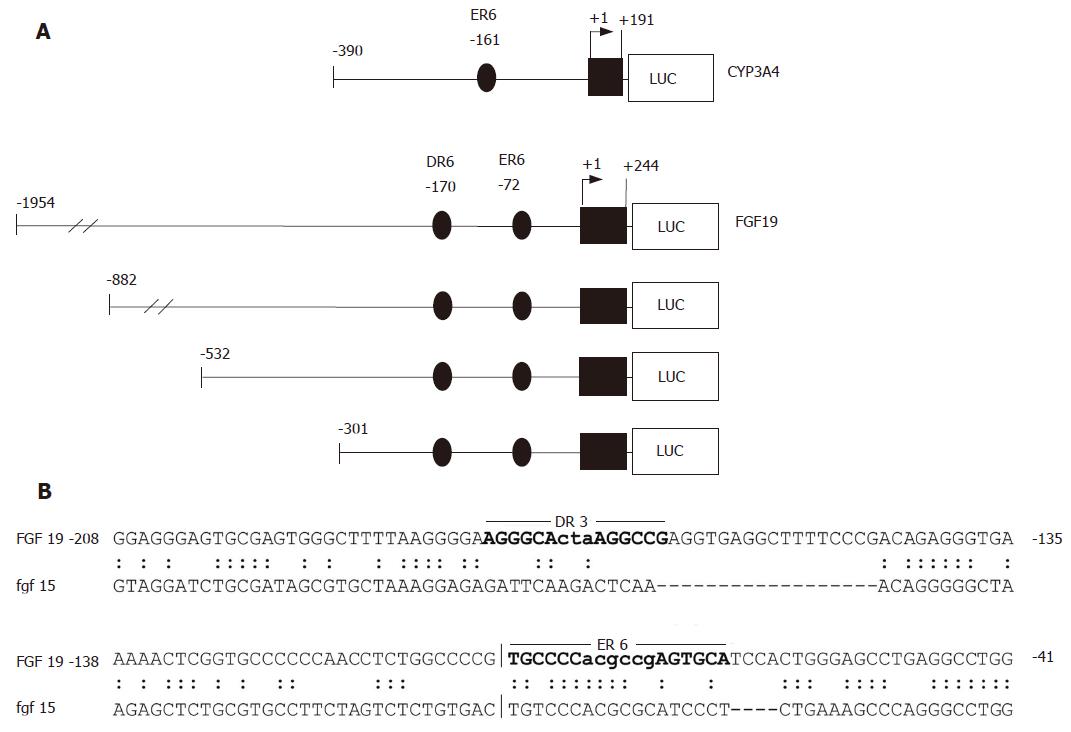
Figure 2 CYP3A4 and FGF19 promoter constructs and sequence alignment of the human and murine FGF19/Fgf15 regulatory regions.
A: CYP3A4 and FGF19 promoter constructs used for transient transfection assays. The positions are calculated relative to the major transcription start site; B: Sequence comparison of homologous regions in the human FGF19 and murine Fgf15 promoters. The locations of the DR3 and ER6 elements identified in the human FGF19 promoter by NUBIScan analysis are indictated by bold letters.
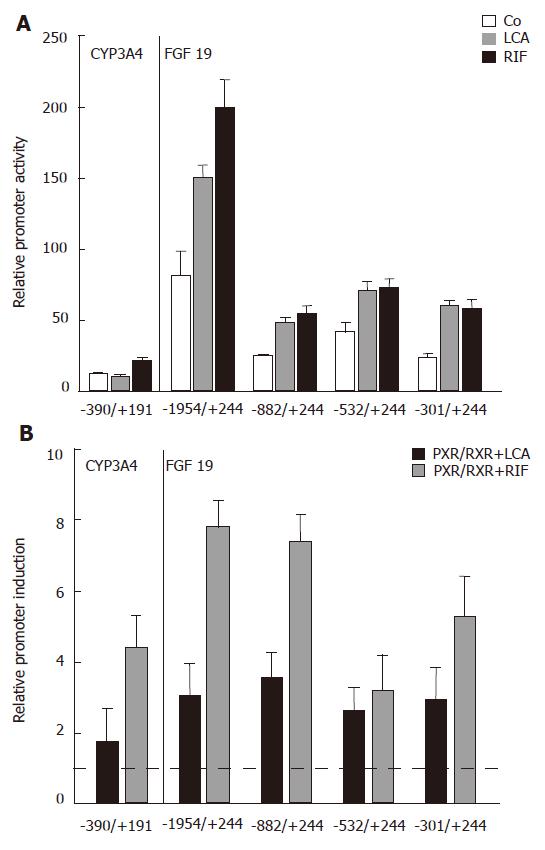
Figure 3 Localization of the LCA/Rif/PXR responsive element in the human FGF19 promoter.
A: Relative CYP3A4 and FGF19 promoter activities of LS174T cells treated with LCA (250 μmol/L) and Rif (10 μmol/L) compared to control treated cells for 24 h (P < 0.05 between LCA and Rif treatment groups and control treated groups); B: Reporter constructs were cotransfected with PXR/RXR or mock plasmids and stimulated with LCA (250 μmol/L) and Rif (10 μmol/L) as indicated for 24 h. Mock transfected and solely LCA/Rif stimulated cells were used for normalization and calculation of relative promoter induction (mean ± SD, Student’s t-test). The dotted line indicates the threshold for significant promoter induction. P < 0.05 between PXR/RXR cotransfected and LCA/Rif treated groups and mock transfected and LCA/Rif treated groups. The used reporter constructs are indicated on the x-axis.
- Citation: Wistuba W, Gnewuch C, Liebisch G, Schmitz G, Langmann T. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR. World J Gastroenterol 2007; 13(31): 4230-4235
- URL: https://www.wjgnet.com/1007-9327/full/v13/i31/4230.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i31.4230