Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. May 21, 2007; 13(19): 2675-2683
Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2675
Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2675
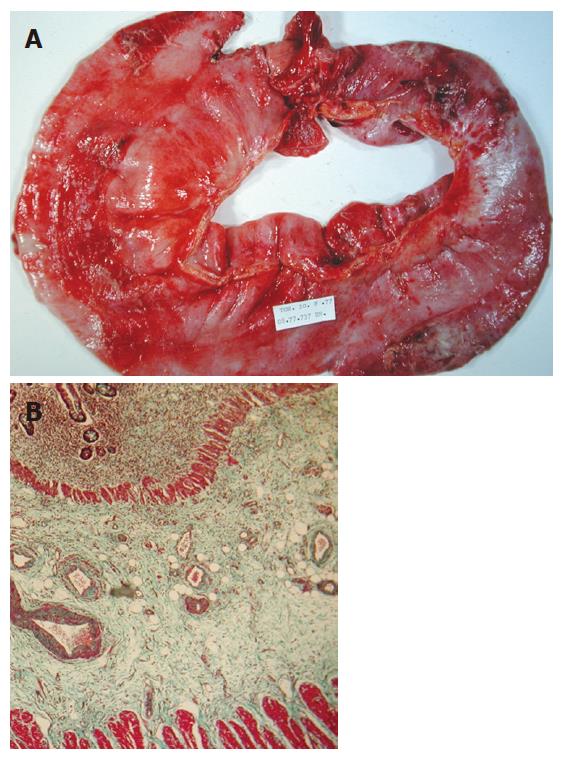
Figure 1 A: Resection of human intestine with radiation-induced fibrosis; B: Bright field photomicrograph showing transmural collagen accumulation as green stain in human radiation enteropathy after Masson’s trichrome staining.
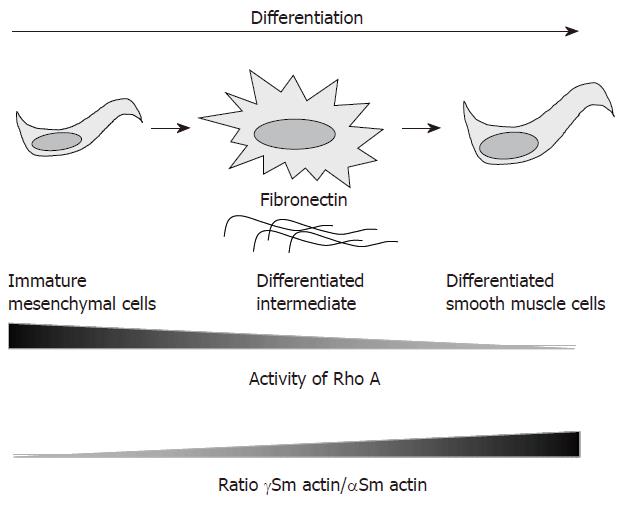
Figure 2 Differentiation of the smooth muscle cells during myogenesis is correlated with increased γSm actin/αSm actin ratio in the intestine[54] and with decreased Rho activity in the lung.
In addition in the lung Beqaj et al[55] isolated a cell exhibiting an intermediate differentiation status after spreading of precursors cells onto fibronectin coating.
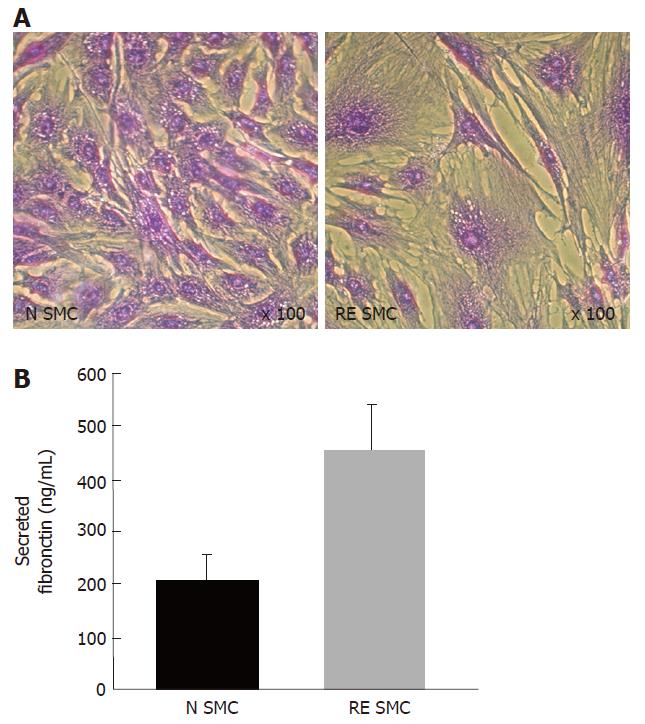
Figure 3 A: Bright field photomicrograph of fibrosis-derived intestinal smooth muscle cells (RE SMC) and normal cells (N SMC) observed after crystal violet staining; B: Fibronectin secretion level in RE SMC and N SMC assessed by ELISA (Chemicon).
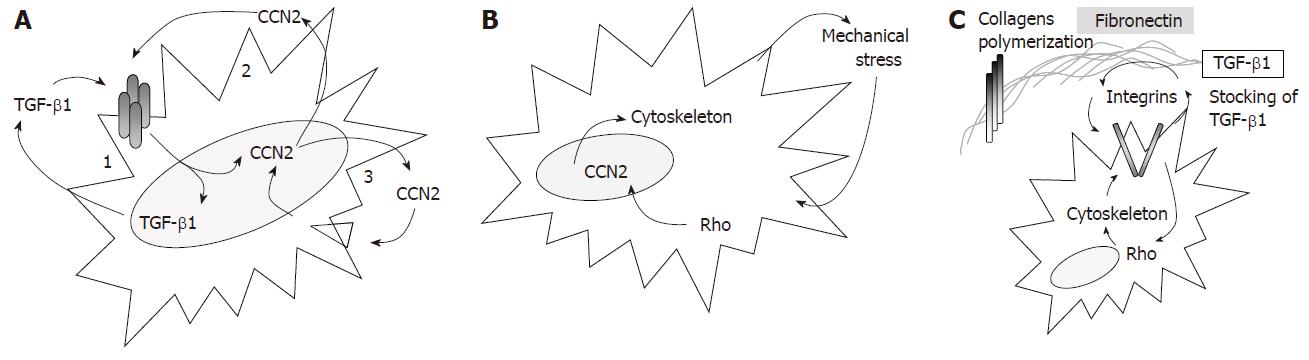
Figure 4 Chronic molecular activation loops, established between mesenchymal cells and its microenvironment, involved in the maintenance of fibrosis.
A: 1-TGF-β1 auto-induction; 3-CCN2 auto-induction; 2-cooperation between CCN2 and TGF-β1; B: Mechanical stress induces Rho pathway activation[73] and CCN2 expression[72]. Rho activation induced modulation of cytoskeleton polymerization and, as a consequence, generated new mechanical stress in the environment; C: Fibronectin is involved in the extra-cellular matrix sequestration of TGF-β1[76], in the polymerization of collagen fibers[77,78] and in the activation of the Rho pathway[79]. The activation of the Rho pathway through integrins, modulated the polymerization of cytoskeleton. This structural cell change produced integrin’s movement at the cell surface and thus modulated the polymerization of fibronectin.
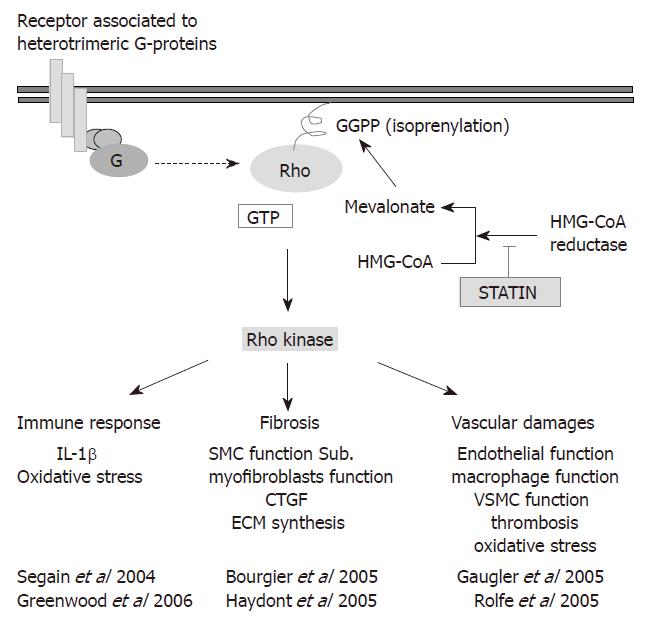
Figure 5 Targeting the Rho/ROCK pathway for normal tissue protection.
Molecular binding on receptor associated to heterotrimeric G-proteins activated Rho protein by a shift between Rho-guanosin diphosphate (Rho-GDP) to Rho-guanosin triphosphate (Rho-GTP). This activation of Rho induced in cascade Rho kinase activation. As a consequence immune response, fibrosis and vascular damage were modulated. Rho protein anchorage to cytoplasmic membrane depends on the isoprenylation. Geranylgeranyl pyrophosphate (GGPP) required for this post-translational modification come from cholesterol metabolism, and may be inhibited by statins.
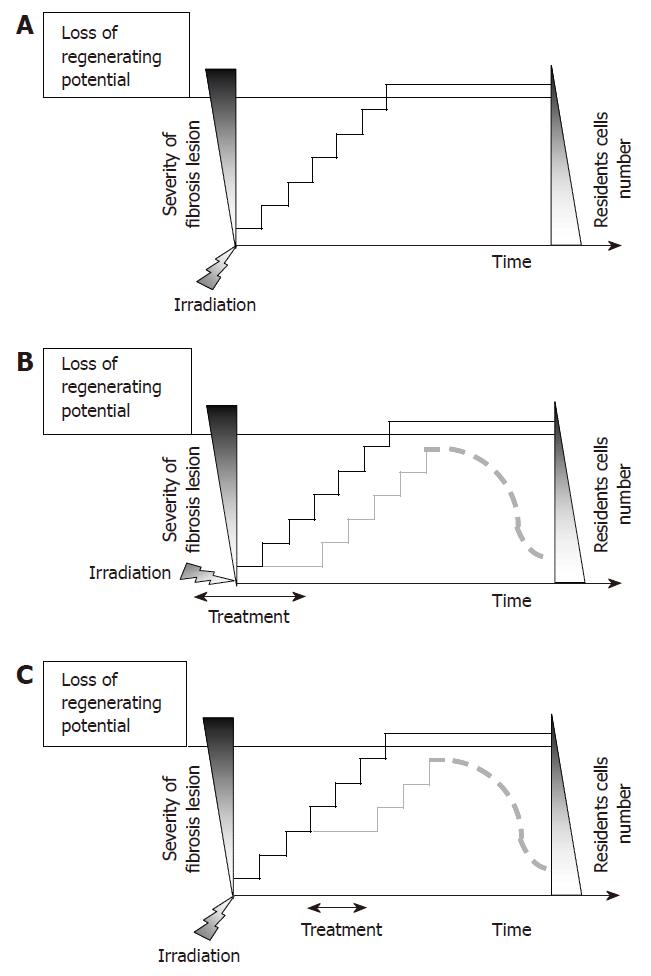
Figure 6 A: Fibrosis is a progressive pathology that involves sequential activation of chronic molecular loops.
Gradation of fibrosis severity correlates with a decreased number of resident cells and decreased the potential of recovery; B: Impact of prophylactic treatment on the development of fibrosis: early inhibition of one or few molecular loops would help to preserve the regenerative potential of the tissue; C: Impact of curative treatment on fibrosis maintenance: inhibition of one or few chronic loop would help to preserve the regenerative potential of the tissue thus leading to tissue recovery.
- Citation: Haydont V, Vozenin-Brotons MC. Maintenance of radiation-induced intestinal fibrosis: Cellular and molecular features. World J Gastroenterol 2007; 13(19): 2675-2683
- URL: https://www.wjgnet.com/1007-9327/full/v13/i19/2675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i19.2675