Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. May 7, 2007; 13(17): 2467-2478
Published online May 7, 2007. doi: 10.3748/wjg.v13.i17.2467
Published online May 7, 2007. doi: 10.3748/wjg.v13.i17.2467
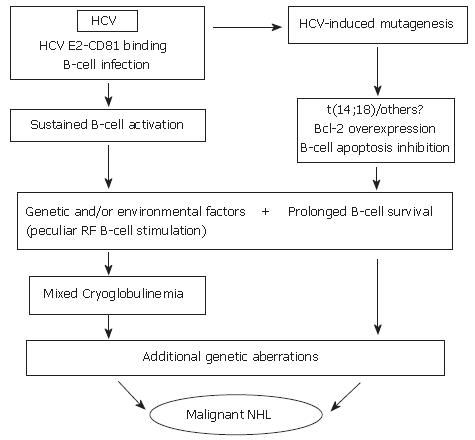
Figure 1 Hypothetical interpretation of the complex relationship between chronic HCV infection, and lymphoproliferative disorders.
During chronic infection, HCV is responsible for sustained B-cell proliferation. Factors favoring polyclonal B lymphoproliferation may include the specific binding of HCV E2 protein to CD 81 (a tetraspannin which, on the B-cell surface, is part of an activating molecular complex lowering the threshold of B-cell activation by specific epitopes)[92,95], as well as the specific activation of reactive T-cells by HCV and cytokines. Favoring factors may also be represented by the persistent infection of B-cells[78,95]. Resulting sustained B-cell proliferation would in turn favor the occurrence of t(14;18) translocation and/or other errors during V(D)J rearrangement processes in germinal centers located in secondary lymphoid organs which, in case of HCV infection, would also include the liver[28]. A more direct role played by HCV lymphatic infection via viral mutagenic properties cannot be excluded[93]. Bcl-2 antiapoptotic protein overexpression in B-cells would result and lead to abnormally prolonged B-cell life. In predisposed subjects, in presence of unknown environmental, viral and/or genetic factors, HCV infection could lead to sustained production of cryoglobulins. These predisposing factors may include the peculiar susceptibility of IgM RF producing B-cells (RF B-cells) to be activated and/or the presence of particular viral variants bearing epitopes capable of selectively activating RF B-cells. It is tempting to hypothesize that the Bcl-2 rearrangement, by inhibiting B-cell apoptosis, may favor the lack of silencing higher affinity, potentially pathological RF B-cells[142], possibly leading to the development of MC syndrome. In turn, abnormal survival of B-cells would favor the acquisition of additional genetic aberrations which might ultimately lead to transformation to a frank B-cell malignancy[33].
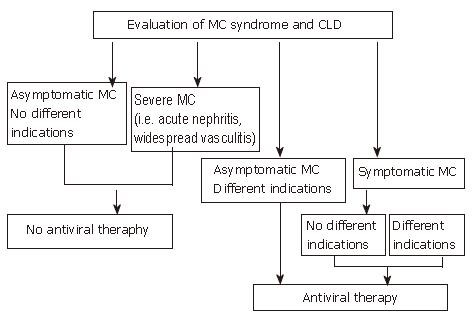
Figure 2 Algorithm for treatment of HCV-positive mixed cryoglobulinemia patients with antiviral therapy according to the evaluation of both mixed cryoglobulinemia syndrome and chronic liver damage.
Available data suggest that antiviral therapy should be considered the first choice treatment in mixed cryoglobulinemia, to be performed even in the absence of different indications, excluding only those patients without symptoms and other indications or patients with too severe manifestations such as acute nephritis or widespread vasculitis. (Zignego AL, Postgraduate Course EASL 2006, Vienna and [34], modified)
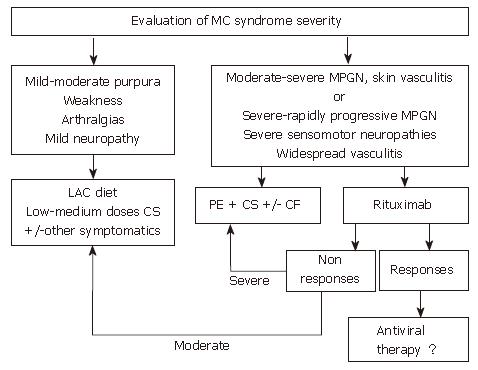
Figure 3 Algorithm for treatment of HCV-positive mixed cryoglobulinemia patients who are non-responders or in whom antiviral treatment is contraindicated or not tolerated.
When antiviral treatment is not indicated this algorithm suggests other approaches for the management of mixed cryoglobulinemia, including anti-inflammatory drugs (first corticosteroids, CS), procedures able to lower the concentration of cryoglobulins such as the low antigen content (LAC) diet or plasma exchange (PE) as well as immunosuppressive drugs (cyclophosphamide, CF). In patients with only mild to moderate syndrome, cycles of therapy with anti-inflammatories, the LAC diet or other symptomatic treatments are suggested. Therapy should be adapted to the single patient and limited in time. In patients with more severe syndromes, cycles of plasma exchange plus corticosteroids and/or immunosuppressive drugs are indicated. The use of rituximab, a selective B-cell suppressor, may be an alternative treatment in some cases where antiviral therapy is initially contraindicated. (Zignego AL, Postgraduate Course EASL 2006, Vienna and [34])
- Citation: Zignego AL, Giannini C, Ferri C. Hepatitis C virus-related lymphoproliferative disorders: An overview. World J Gastroenterol 2007; 13(17): 2467-2478
- URL: https://www.wjgnet.com/1007-9327/full/v13/i17/2467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i17.2467