Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 14, 2006; 12(46): 7397-7404
Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7397
Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7397
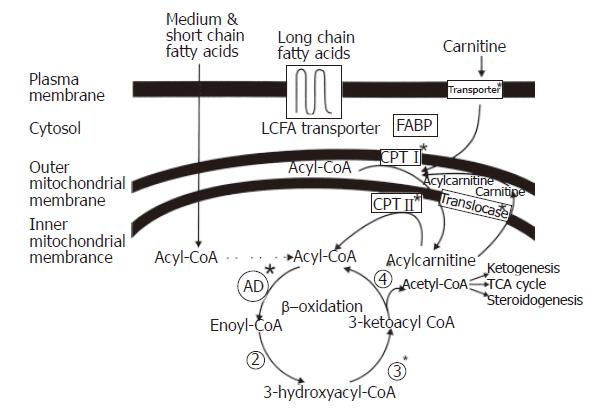
Figure 1 Fatty Acid Import into the Mitochondria and Fatty Acid β-oxidation.
Long chain fatty acids are actively transported across the plasma membrane, esterified to coenzyme A, carried by fatty acid binding proteins through the cytoplasm to the mitochondria and translocated across the mitochondrial membranes by the carnitine shuttle into the mitochondrial matrix. The fatty acid is subsequently cleaved into two shorter carbon entities by the β-oxidation spiral.
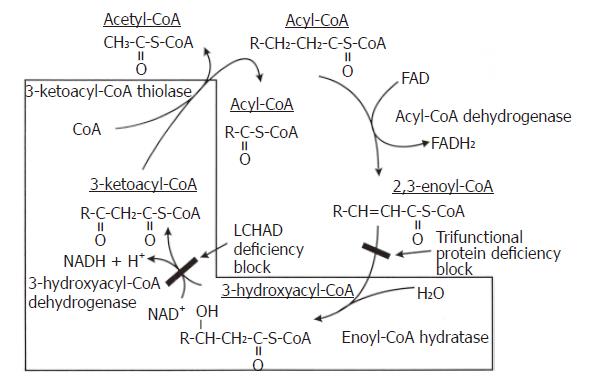
Figure 2 The Biochemistry of TFP and LCHAD Deficiencies.
The last three reactions of the mitochondrial fatty acid β-oxidation spiral where the trifunctional protein catalyzes long chain fatty acids substrates. In isolated LCHAD deficiency, the pathway is blocked after the enoyl Co-A hydratase reaction and before the 3-hydroxyacyl Co-A dehydrogenase reaction, causing the accumulation of medium- and long-chain 3-hydroxy fatty acids and their metabolites. In complete TFP deficiency, the pathway is blocked after the acyl Co-A dehydrogenase reaction and before the enoyl Co-A dehydrogenase reaction causing the accumulation of straight chain fatty acids and their metabolites.
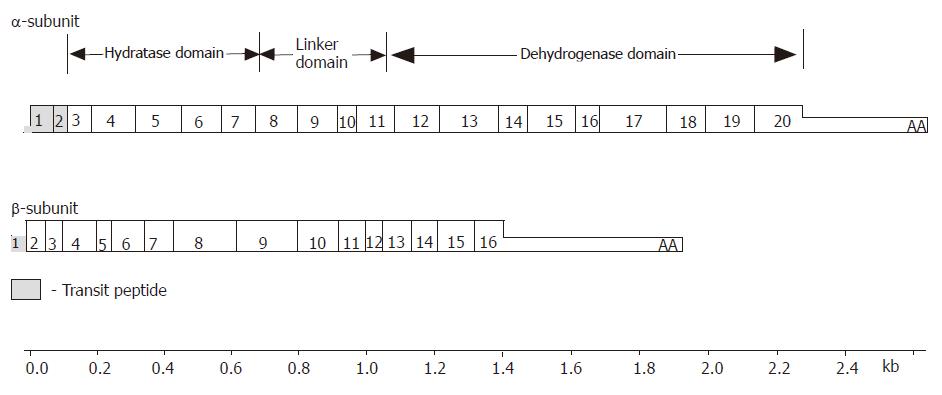
Figure 3 The structure of MTP subunit cDNA’s.
The MTP α-subunit contains 20 exons. Exons 1 and 2 encode the transit peptide that directs uptake into the mitochondria which is proteolytically cleaved during translocation into the mitochondria. Exons 3-8 encode the N-terminus of the mature α-subunit containing the long-chain 2,3-enoyl-CoA hydratase activity. Exon 9 encodes the region known as a linker domain. Exons 11-20 encode the C-terminus of the mature α-subunit containing the LCHAD activity. The MTP β-subunit has 16 exons that encode the long-chain 3-keto-acyl CoA thiolase activity.
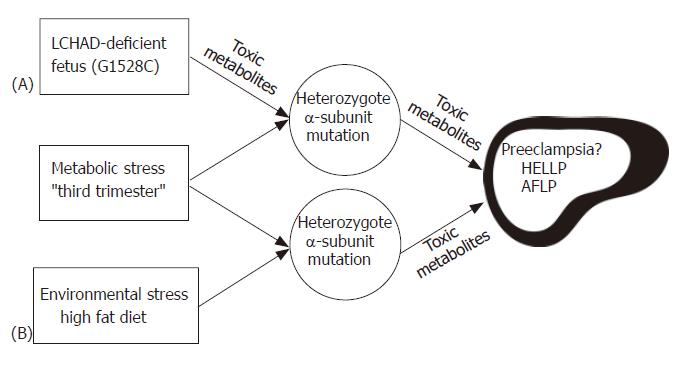
Figure 4 Hypothesis illustrating the possible role of fetal and maternal MTP mutations in developing AFLP.
Carrying an LCHAD deficient fetus (A) is the major determining factor in the development of maternal illness. Hepatotoxic metabolites produced by the fetus and/or placenta may cause liver disease in the obligate heterozygous mother when combined with the metabolic stress of the third trimester. Environmental stress (B) may lead to the further accumulation of toxic metabolites in the genetically susceptible mother causing maternal liver disease.
- Citation: Ibdah JA. Acute fatty liver of pregnancy: An update on pathogenesis and clinical implications. World J Gastroenterol 2006; 12(46): 7397-7404
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7397