Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 21, 2006; 12(27): 4310-4317
Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4310
Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4310
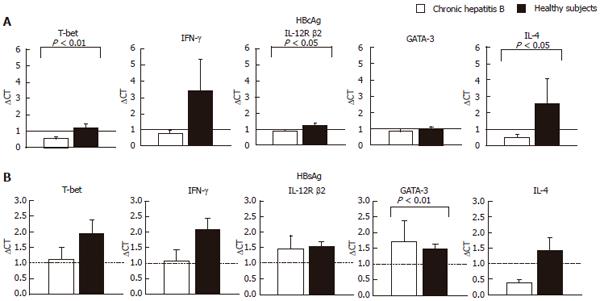
Figure 1 Comparison of levels of mRNAs for T-bet and GATA-3 after stimulation with HBsAg and HBcAg with mRNAs for IFN-gamma, IL-10 and IL-4.
Total cellular RNA was extracted from CD4+ T cells after the stimulation of PBMCs with HBcAg (10 μg/mL) or HBsAg (29 μg/mL) for 24 h. A: HBcAg stimulation; B: HBsAg stimulation. Levels of mRNA for T-bet, GATA-3, IFN-γ, IL-12R β2 and IL-4 were quantified by TaqMan PCR. GAPDH was used as an internal control. Relative amount of target mRNA was calculated using comparative CT method. The expression level of mRNAs of the non-stimulated sample in each subject is represented as 1.0 and relative amount of target mRNA in a stimulated sample was calculated using the as following formula: relative amount = 2-ΔΔCT, where ΔΔCT was given by subtracting ΔCT (non-stimulated cells) from ΔCT (stimulated cells). The ΔCT value was determined by subtracting the GAPDH CT value from the target CT value. The validation experiments were performed in advance for all the target mRNAs to demonstrate that efficiency of each target and GAPDH are approximately equal.
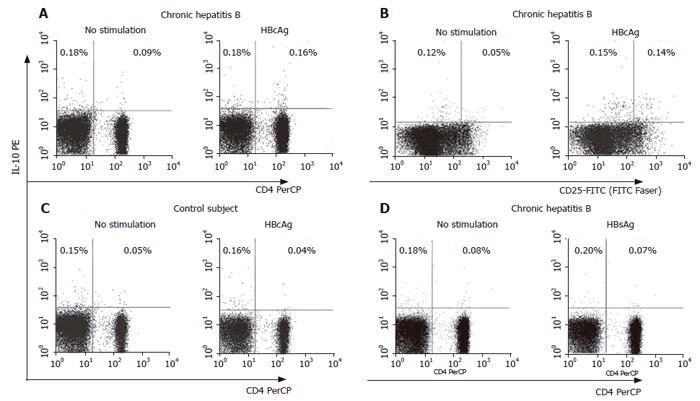
Figure 2 FACS analysis of HBcAg-specific production of IL-10 in patients with hepatitis B.
Cellular source of HBcAg-specific production of IL-10 was identified by staining for IL-10-secretion (PE-labeled), anti-CD3-PerCP, anti-CD4-PerCP and anti-CD25-FITC. Representative dot plots of IL-10-secreting CD4+ T cells in a patient with CHB (A) and IL-10-secreting CD4+CD25high T cells in a patient with CHB (B). For the control, IL-10-secreting cells in a healthy subject with HBcAg stimulation (C) and in a patient with CHB with HBsAg stimulation (D) were also shown. Numbers shown in the dot plots indicate percentage of the cells in the quadrant region.
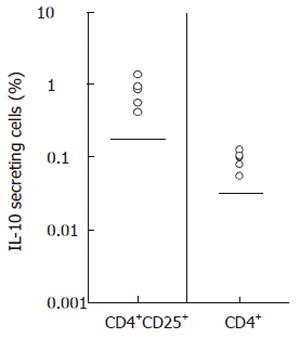
Figure 3 Increased populations of HBcAg-specific IL-10-producing CD4+ or CD4+CD25high T cells in patients with chronic hepatitis B.
Population of IL-10 secreting cells in CD4+ T cells and in CD4+CD25+ T cells was evaluated in patients with CHB. Frequencies of HBcAg-specific IL-10 secreting cells were calculated by subtracting percentage in non-stimulated samples from percentage in HBcAg-stimulated samples. Upper limits of normal subjects (mean ± 2SD of 5 subjects) were shown by straight lines in the plots (0.14% for CD4+CD25+ cells and 0.027% for CD4+ cells). A FITC faser kit (BD Bioscience Pharmingen) was used in some experiments of ease separation of positive events by enhancing fluorescence intensity.
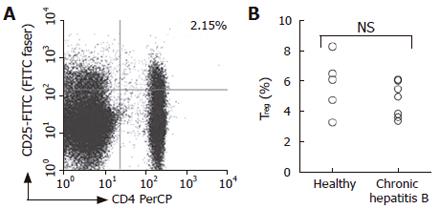
Figure 4 Comparison of CD4+CD25high T cell population between patients with hepatitis B and healthy subjects.
The cells that express CD4, CD25high and CTLA-4 were identified by flow cytometry. Representative dot plots of an ex vivo sample of a patient with CHB is shown (A), numbers shown in the dot plot indicates percentage of cells in the quadrant lesion. Percentage of CD4+CD25+ T cells was shown for patients with CHB and healthy subjects (B).
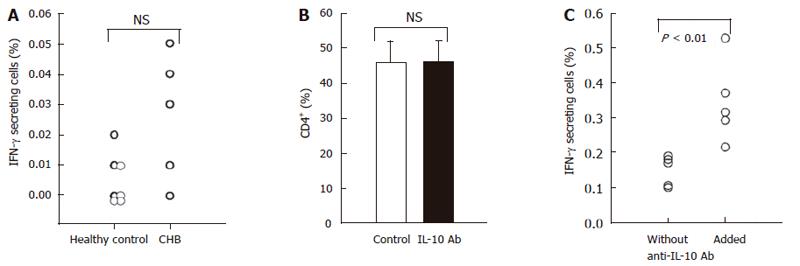
Figure 5 Addition of neutralizing anti-IL-10 antibody restores HBcAg-specific production of IFN-γ by CD4+ in patients with hepatitis B.
PBMCs obtained from 5 patients with CHB and 7 healthy subjects were stimulated with HBcAg (10 μg/mL) for 9 h and thereafter cells were stained for IFN-γ-secretion (PE) and anti-CD4-PerCP to determine the population of HBcAg-specific TH1 being identified as IFN-γ+ cells in CD4+ T cells (A). Anti-IL-10 neutralizing antibody or isotype-matched control antibody were added to the culture during stimulation with HBcAg. The addition of anti-IL-10 antibody did not affect the percentage of CD4+ T cells (B). In culture with anti-IL-10 antibody, numbers of HBcAg-specific TH1 were significantly higher than those in culture with a control antibody (C).
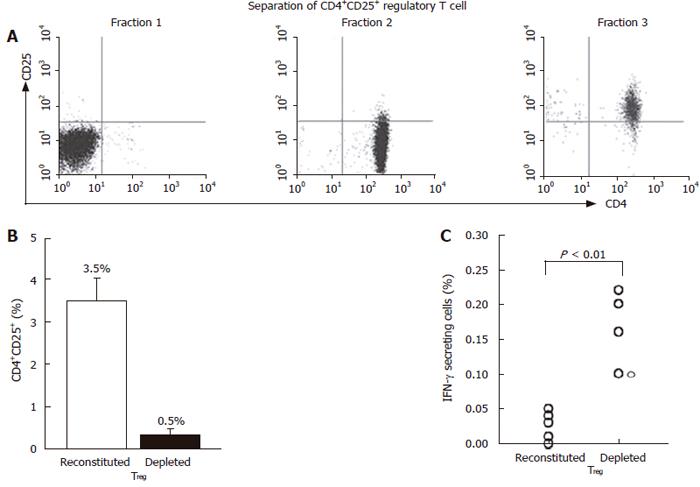
Figure 6 Depletion of CD4+CD25+ T cells from PBMCs increases HBcAg-specific production of IFN-γ in patients with hepatitis B.
Using the differential expression of CD4 and CD25, cells were separated into 3 fractions; fraction 1 consisted of CD4- cells, fraction 2 consisted of CD4+CD25- cells and fraction 3 consisted of CD4+CD25+ cells (A). Thereafter, 2 sets of lymphocyte preparations were reconstituted by remixing fractions 1, 2 and 3 or by remixing fractions 1 and 2 (B). They were stimulated with HBcAg to finally stain a CD4+IFN-γ+ population (C).
- Citation: Kondo Y, Kobayashi K, Ueno Y, Shiina M, Niitsuma H, Kanno N, Kobayashi T, Shimosegawa T. Mechanism of T cell hyporesponsiveness to HBcAg is associated with regulatory T cells in chronic hepatitis B. World J Gastroenterol 2006; 12(27): 4310-4317
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4310