Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 7, 2006; 12(25): 3989-3993
Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.3989
Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.3989
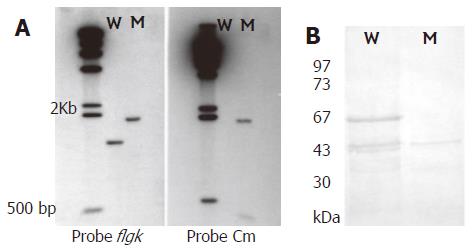
Figure 1 A: Southern blotting of genomic DNA from H pylori 250 and its flgK mutant digested with HindIII.
The probes used were specific for the flgK gene, chloramphenicol resistance (Cm) gene and λ DNA. Lane W, wild-type strain; lane M, mutant; λHindIII marker used as a molecular size standard; B: Western blotting of anti-r-FlgK polyclonal antibodies to confirm the phenotype of flgK isogenic mutant (absence of 67 kDa band). Lane W, wild-type strain. Lane M, mutant. Low molecular weight protein markers.
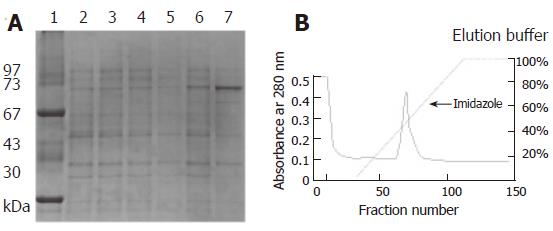
Figure 2 The r-FlgK (73 kDa) can be expressed under IPTG induction by SDS-PAGE.
A: Lane 1: Low molecular protein marker, Lane 2: E. coli BL21 strain, Lane 3: E. coli BL21 strain, IPTG induction, Lane 4: pET-30b/ E. coli BL21 strain, Lane 5: pET-30b/ E. coli BL21 strain, IPTG induction, Lane 6: flgK/ E. coli BL21 strain, Lane 7: flgK/ E. coli BL21 strain, IPTG induction; B: The purification of r-FlgK protein by Ni2+-chelating chromatography column.
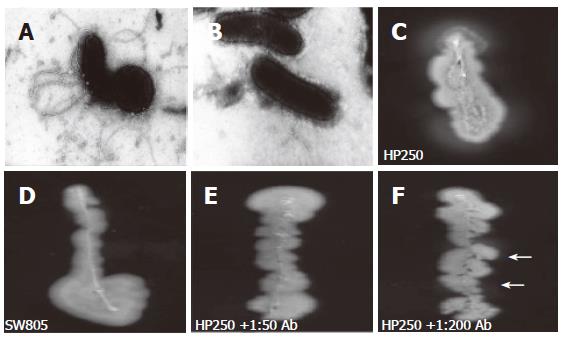
Figure 3 The electron microscopy disclosed wild-type H pylori had full-length sheathed flagella (A), which was not observed in the flgK mutant (B).
In vitro motility testing disclosed halos of wild-type strain (C), whereas the mutant was without halo (D). An evident halo (arrows) indicated a poorer inhibition of the motility of wild-type H pylori by adding 1:200 anti-r-FlgK polyclonal antibody (F) than by adding 1:50 of the antibody (E).
-
Citation: Wu JJ, Sheu BS, Huang AH, Lin ST, Yang HB. Characterization of
flgK gene and FlgK protein required forH pylori Colonization-from cloning to clinical relevance. World J Gastroenterol 2006; 12(25): 3989-3993 - URL: https://www.wjgnet.com/1007-9327/full/v12/i25/3989.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.3989