Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. May 7, 2006; 12(17): 2641-2655
Published online May 7, 2006. doi: 10.3748/wjg.v12.i17.2641
Published online May 7, 2006. doi: 10.3748/wjg.v12.i17.2641
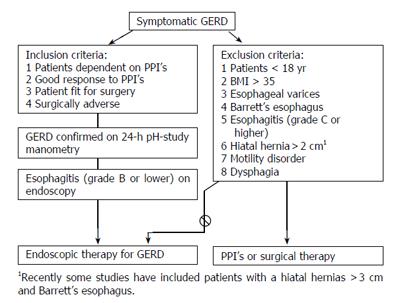
Figure 1 Inclusion/exclusion criteria for endoluminal therapies of GERD.
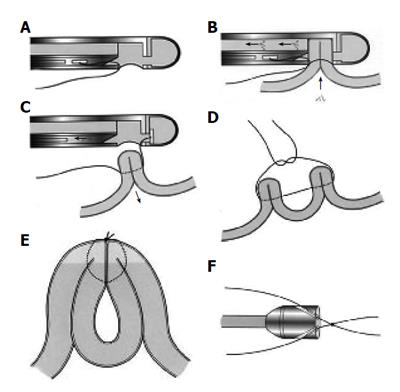
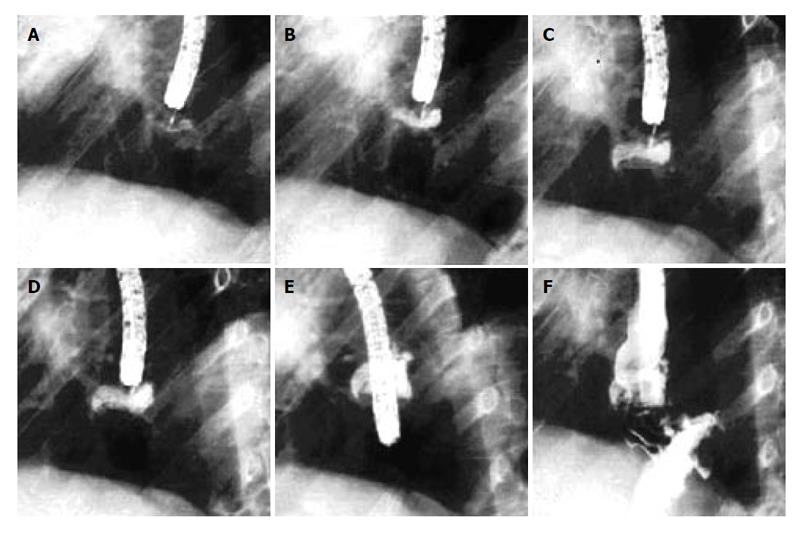
Figure 2 A: Drawing of the suturing device which is equipped with a vacuum chamber and a hollow needle in which there is a suture attached to a tag; B: Tissue is drawn into the chamber by suction.
The needle passes through the tissue and the tag is captured in the distal chamber; C: The suction is then released; D: The procedure is repeated on an adjacent piece of tissue; E: On tightening the knot, the pieces of tissues are approximated; F: The knot, tied outside the animal, is advanced by a knot pusher which is attached to the tip of the endoscope.
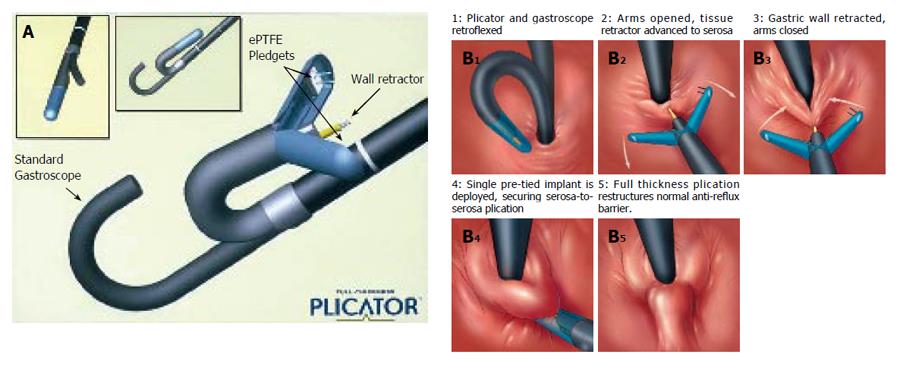
Figure 3 A: The NDO plicator mounted on a small diameter endoscope; B: Schematic representation of the procedure for application of a full-thickness plication by NDO plicator.
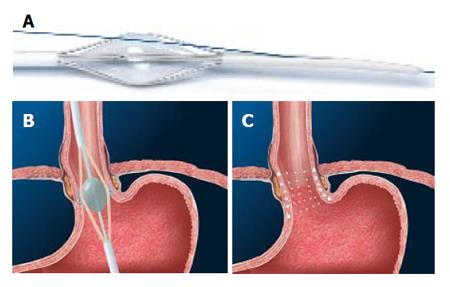
Figure 4 Syntheon device.
Figure 5 A: The Stretta catheter with guidewire; B: The balloon assembly with struts and electrodes extending into the muscularis propria; C: A total of 56 lesions are applied as seen in this diagram.
Figure 6 Enteryx injection under fluoroscopic control with a resultant prosthetic cuff.
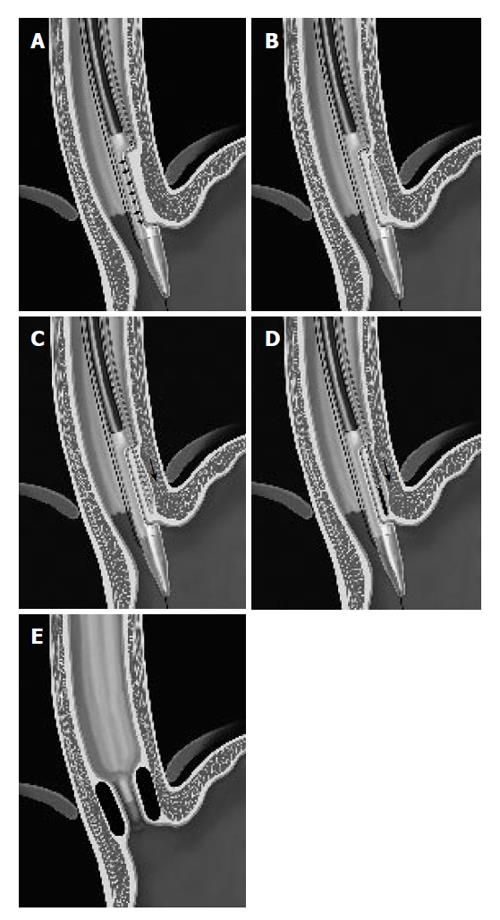
Figure 7 Gatekeeper procedure.
Sequence of the procedure: A. Stabilization B. Create space C. Access space D. Delivery E. Gatekeeper.
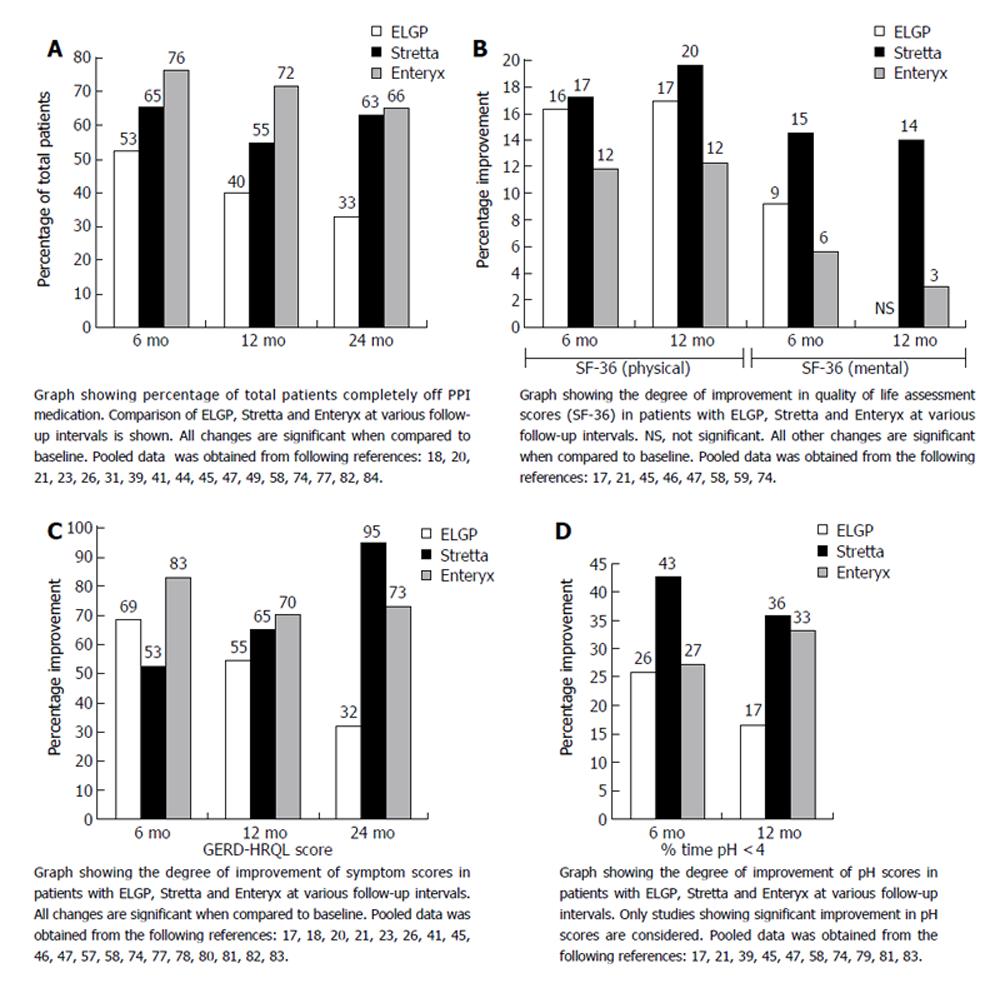
Figure 8 A: Percentage of total patients off PPI’s at 6, 12 and 24 mo post-procedure; B: Improvement in quality of life scores (SF-36), both physical and mental, at various follow-up intervals; C: Degree of symptomatic improvement at various follow-up intervals; D: Improvement in % time pH <4 at 6- and 12- mo follow-up.
- Citation: Iqbal A, Salinas V, Filipi CJ. Endoscopic therapies of gastroesophageal reflux disease. World J Gastroenterol 2006; 12(17): 2641-2655
- URL: https://www.wjgnet.com/1007-9327/full/v12/i17/2641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i17.2641