Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 28, 2006; 12(12): 1859-1864
Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1859
Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1859
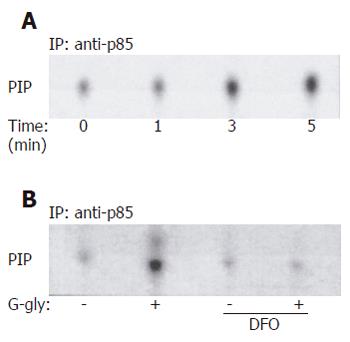
Figure 1 G-gly-induced PI3K (A) and G-gly (B) activation.
HCT116 cells were stimulated with G-gly (10 pmol/L) for the indicated time. PI3K activity was determined in an immune complex kinase assay as described in Methods. The specificity of G-gly activation was accessed by pretreating the cells with ferric chelator DFO (1µmol/L). Representative autoradiograms from at least three separate experiments are shown.
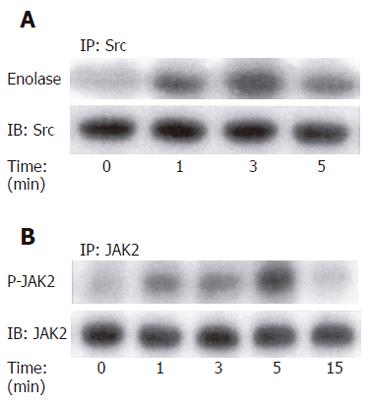
Figure 2 G-gly-induced p60-Src (A) and JAK2 (B) activation.
HCT116 cells were stimulated with G-gly (10 pmol/L) for the indicated time. p60-Src and JAK2 activities were determined in an immune complex kinase assay as described in Methods. Immunoprecipitated proteins were also analysed by Western-blot using the anti-p60-Src and anti-JAK2 antibody respectively. Representative autoradiogram from at least three separate experiments is shown.
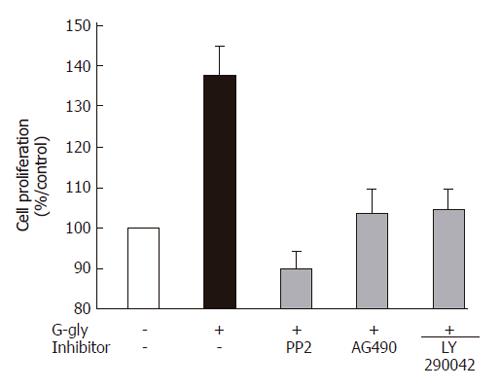
Figure 3 Role of PI3K, Src or JAK2 pathways in human colonic tumour cell proliferation induced by G-gly.
Serum-starved HCT116 cells were treated with G-gly for 48h in the presence or absence of the indicated inhibitor (10 µmol/L). The proliferation was determined as described in Methods. Each point represents data from triplicate and is representative of 5 independent experiments. Data are presented as means ± SE.
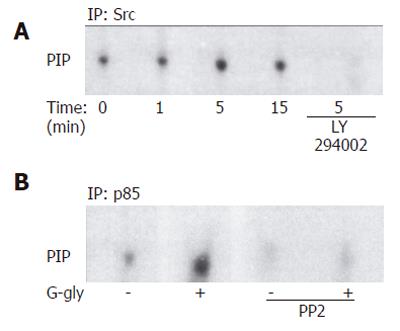
Figure 4 Involvement of p60-Src (A) and PI3K (B) activation in response to G-gly.
HCT116 cells pretreated with or without 10 µmol/L of LY290042 and anti-p85 antibody were stimulated (+) or not (-) with glycine-extended gastrin (10 pmol/L) for the indicated time. After immunoprecipitation of cell lysates with anti-p60-Src and anti-p85 antibody, precipitates were assayed for PIK activity using inositol phosphate as an exogenous substrate. Lipids were separated by thin layer chromatography and autoradiographied. The result shown is representative from five and three separate experiments.
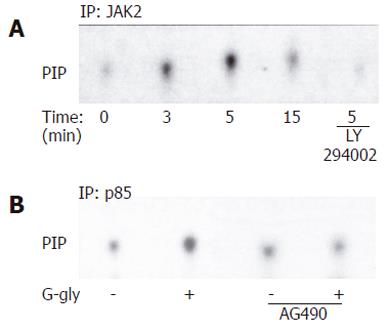
Figure 5 Involvement of PI3K (A) and JAK2 (B) activation in response to G-gly.
HCT116 cells pretreated with or without 10µmol/L of LY290042 and were 10µmol/L of AG 40, stimulated (+) or not (-) with glycine-extended gastrin G-gly (10 pmol/L) for the indicated time. After immunoprecipitation of cell lysates with anti-JAK2 and anti-p85 antibody, precipitates were assayed for PIK activity using inositol phosphate as an exogenous substrate. Lipids were separated by thin layer chromatography and autoradiographied. The result shown is representative from five separate experiments.
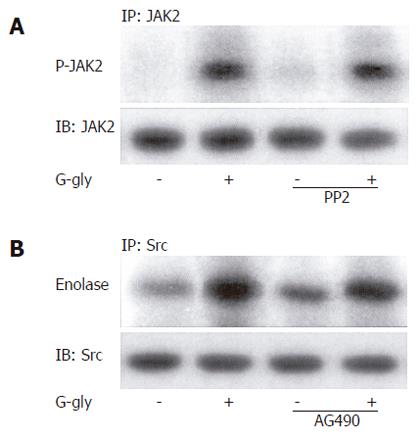
Figure 6 No involvement of p60-Src (A) and JAK2 (B) activation in response to G-gly.
Cells pretreated with or without PP2 and AG 490 (10 µmol/L), were stimulated (+) or not (-) by G-gly (10 pmol/L) for 5 min. JAK2 and p60-Src kinase activities were determined by immune complex kinase assay using anti-JAK2 and anti-p60-Src antibodies as described in Materials and Methods. Proteins were separated by SDS-PAGE and JAK2 autophosphorylation was detected by autoradiography. A representative autoradiogram from at least three separate experiments is shown.
- Citation: Ferrand A, Kowalski-Chauvel A, Pannequin J, Bertrand C, Fourmy D, Dufresne M, Seva C. Glycine-extended gastrin activates two independent tyrosine-kinases in upstream of p85/p110 phosphatidylinositol 3-kinase in human colonic tumour cells. World J Gastroenterol 2006; 12(12): 1859-1864
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1859.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1859