Copyright
©The Author(s) 2004.
World J Gastroenterol. Jan 15, 2004; 10(2): 234-237
Published online Jan 15, 2004. doi: 10.3748/wjg.v10.i2.234
Published online Jan 15, 2004. doi: 10.3748/wjg.v10.i2.234
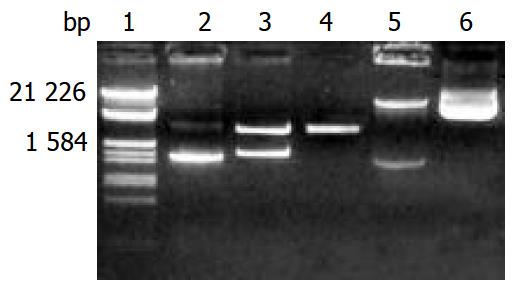
Figure 1 Electrophoresis identification of pGEM-CYP2D6 and pREP9-CYP2D6 recombinants.
Lane 1: Marker (λ/EcoR I and Hind III), 2: PCR products of CYP2D6 (1.543 kb), 3: Recombi-nant of pGEM-CYP2D6 digested by Xho I and BamH I, 4: pGEM-T vector (3 kb), 5: Recombinant of pREP9-CYP2D6 digested by Xho I and BamH I, 6: pREP9 vector (10.5 kb).
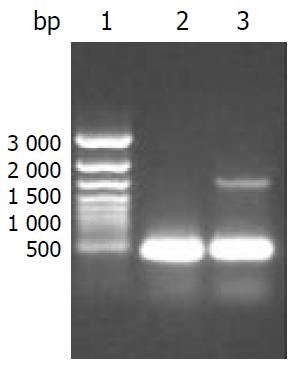
Figure 2 Identification of CYP2D6 mRNA expression in HepG2-CYP2D6 and HepG2 cells by RT-PCR with beta-actin as internal control.
Lane 1: 1 kb ladder marker, 2: RT-PCR prod-ucts of HepG2 cells showing a 462 bp of beta-actin, 3: RT-PCR products of HepG2-CYP2D6 cells showing a 462 bp of beta-actin and 1.5 kb of CYP2D6.
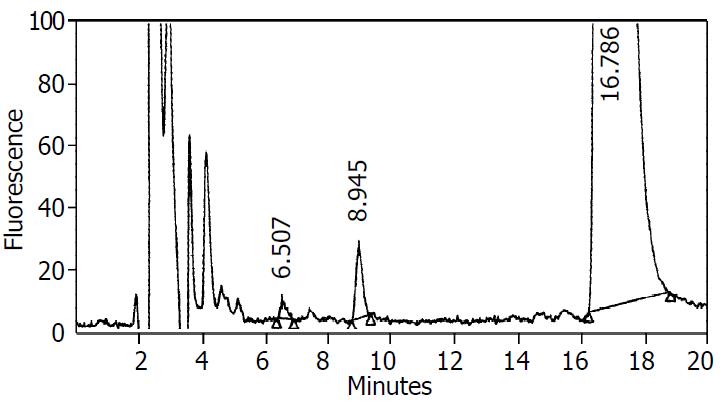
Figure 3 Representative chromatograms of metabolites in supernatant.
10 μL of supernatant was injected into a Water HPLC equipped with a Shimadzu RF-535 fluorescence detector. A CLC phenyl column (15 cm×4.5-mm i.d.) was used to sepa-rate the metabolites. The mobile phase consisted of a mixture of 30% acetonitrile, 1% acetic acid, and 0.05% triethylamine in water. The flow rate through the column at 25 °C was 0.75 ml·min-1. The excitation and emission wavelengths of the fluorescence de-tector were 285 nm and 310 nm, respectively. The retention times for dextrophan and dextromethorphan were 6.5 min and 16.8 min, respectively. The retention time of an unidentified metabolite was 8.9 min.
- Citation: Zhuge J, Yu YN, Wu XD. Stable expression of human cytochrome P450 2D6*10 in HepG2 cells. World J Gastroenterol 2004; 10(2): 234-237
- URL: https://www.wjgnet.com/1007-9327/full/v10/i2/234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i2.234