Copyright
©The Author(s) 2004.
World J Gastroenterol. Jun 15, 2004; 10(12): 1769-1774
Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1769
Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1769
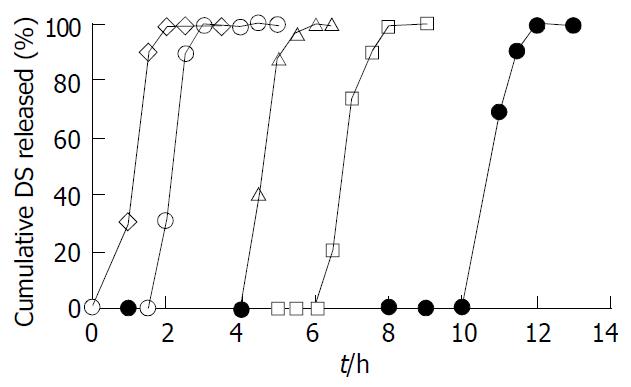
Figure 1 Effect of different coating levels on the DS release from the coated tablets in distilled water.
The tablets were coated at five levels of 3% (◇), 5% (○), 7% (△), 9% (□●), 11%(×) (w/w, total solid applied) (n = 6).
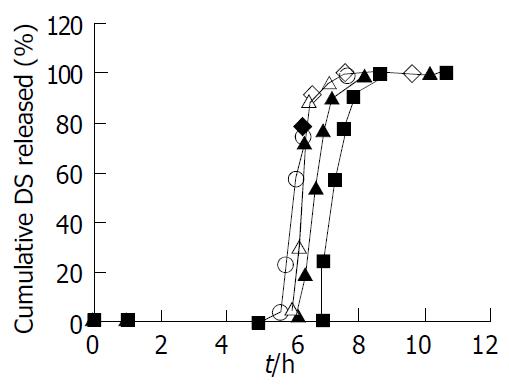
Figure 2 Effect of pH of the dissolution medium on DS release from the coated tablets.
The tablets were coated at 7.5% (w/w, total solid applied) level (n = 6). pH1.0 (◇), pH2.0 (■), pH5.0 (▲), pH6.8 (○), pH7.6 (△) of the dissolution media.
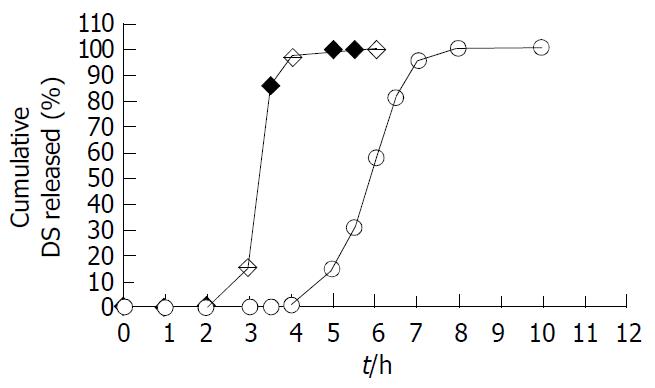
Figure 3 DS dissolution profiles of test coated tablets (○) and the reference enteric coated tablets (◆) (n = 6).
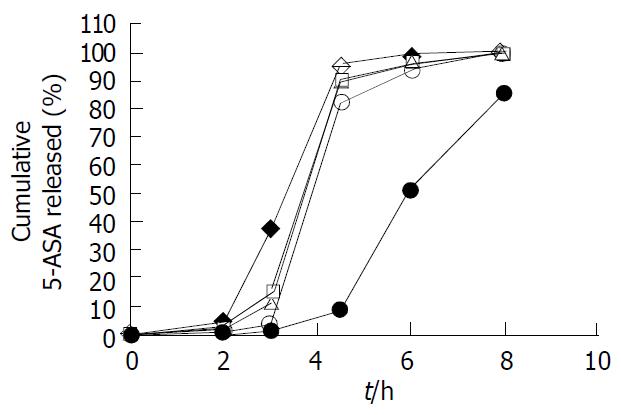
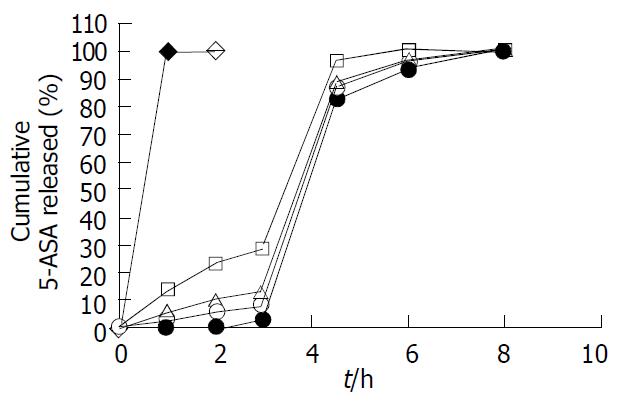
Figure 4 5-ASA release profile of pellets coated at 25% level with various Eudragit® L100-Eudragit® S100 combination ra-tios of 1:0 (), 4:1 (□), 1:1 (△), 1:4 (○) and 0:1 (● ) (w/w), using the sequential pH change method (n = 6).
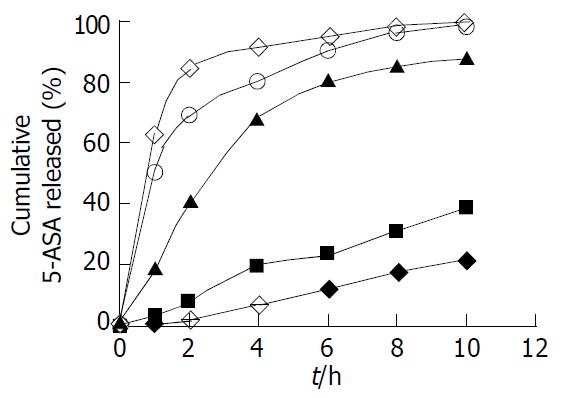
Figure 5 5-ASA release profile of pellets coated at 5 levels of uncoated (◆), 5% (□), 10% (△), 15% (○), 25% (●) (w/w, total solid applied) with Eudragit® L100-Eudragit® S100 combinations of 1:4 (w/w), using the sequential pH change method (n = 6).
Figure 6 Effect of pH of the dissolution medium on 5-ASA release of coated pellets coated at 25% level with Eudragit® L100-Eudragit® S100 combination ratios of 1:4 (w/w) (n = 6).
PH1.2 (◆), pH6.0 (■), pH6.8 (▲), pH7.2 (○), pH7.5 (◇) of the dissolution media.
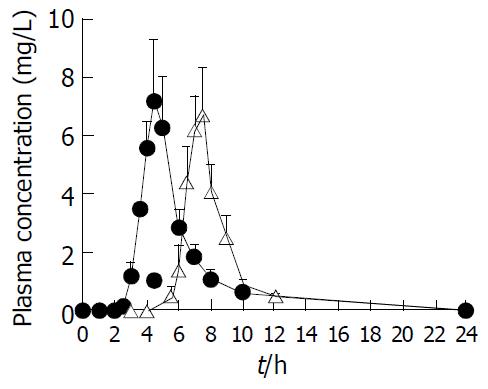
Figure 7 DS plasma concentration-time profiles after oral ad-ministration of coated formulation (△) and reference enteric formulation (●) in dogs at a dose of 100 mg/body.
Each value represents mean ± SE (n = 6).
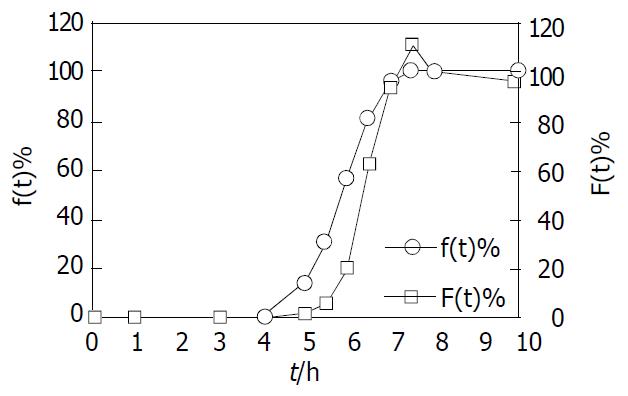
Figure 8 Comparison between the cumulative DS release per-centage in vitro f(t) and the absorption percentage in vivo F(t) of coated formulation (n = 6).
- Citation: Cheng G, An F, Zou MJ, Sun J, Hao XH, He YX. Time- and pH-dependent colon-specific drug delivery for orally administered diclofenac sodium and 5-aminosalicylic acid. World J Gastroenterol 2004; 10(12): 1769-1774
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1769