Published online Jan 12, 2023. doi: 10.13105/wjma.v11.i1.29
Peer-review started: August 25, 2022
First decision: October 4, 2022
Revised: October 13, 2022
Accepted: November 23, 2022
Article in press: November 23, 2022
Published online: January 12, 2023
The recent and still ongoing pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entailed various long-term complications, including post-infectious cholangiopathy.
To identify the available studies concerning post-coronavirus disease 2019 (COVID-19) cholangiopathy.
An extensive bibliographical search was carried out in PubMed and in Cochrane Library to identify the articles (retrospective and prospective studies, cohort studies, case series and case reports) published between January 1, 2020 and August 22, 2022, using both MeSH terms and free-language keywords: cholangiopathy; COVID-19; post-COVID-19 cholangiopathy; SARS-CoV-2.
Thirteen studies fulfilled the inclusion criteria, which included 64 patients suffering from this condition. The patients were male in 82.8% of cases. Liver transplant was executed in 6 patients and scheduled in 7 patients, while 2 patients refused the surgical approach. Therefore in 23.4% of the cases, performing this procedure appeared to be necessary.
This review has revealed that generally the involvement of the liver in the course of SARS-CoV-2 infection is mild and transient, inducing cholestasis of cholangiocytes but can also be severe enough to cause organ failure in some cases.
Core Tip: As severe acute respiratory syndrome coronavirus 2 infection keeps spreading, its long-term complications, like cholangiopathy, will manifest. Post-coronavirus disease 2019 (COVID-19) cholangiopathy is most commonly identified in patients hospitalized in the intensive care unit and shows histological characteristics reminiscent of secondary sclerosing cholangitis. Post-COVID-19 cholangiopathy represents a serious complication that may evolve into liver failure, even requiring transplant.
- Citation: Zippi M, Fiorino S, Hong W, de Biase D, Gallo CG, Grottesi A, Centorame A, Crispino P. Post-COVID-19 cholangiopathy: A systematic review. World J Meta-Anal 2023; 11(1): 29-37
- URL: https://www.wjgnet.com/2308-3840/full/v11/i1/29.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i1.29
It is well known that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the disease named coronavirus disease 2019 (COVID-19), can induce liver damage in addition to the prevailing respiratory diseases[1]. This pathogen determines gastrointestinal symptoms, especially hepatic, with a multifactorial modality: direct damage, intestinal translocation, drug hepatotoxicity and immune-mediated inflammation secondary to the “cytokine storm”[2-4].
The first mechanism described is due to the presence of angiotensin converting enzyme-2 (ACE-2) receptors expressed on the liver cells, in particular on the epithelial cells of cholangiocytes[5,6]. To the best of our knowledge, the first pathological description of the liver was reported in 2020 by Xu et al[7], who described a mild lobular and portal inflammation, thus exhibiting direct liver damage sustained by this virus. The reported incidence of liver injury ranges between 14.8% and 53.0% of infected patients, of which 2%-11% are suffering from known hepatic pathologies (nonalcoholic fatty liver disease, chronic viral hepatitis, immune-mediated liver disease and alcoholic hepatitis)[7,8]. The hepatic symptoms characterized by an increase of the transaminases and/or of the cholestasis indices are widely described in the literature and tend to appear during the course of the infection and decrease at the end of the disease course[9].
In particular, an increase in serum gamma-glutamyl transferase (GGT) levels has been present in 27.9% of severe forms of COVID-19, suggesting an ongoing damage to the cholangiocytes[7,10]. Cholestasis is induced by high simultaneous values of GGT and alkaline phosphatase (ALP)[9]. In 2021, Roth et al[11] described a new hepatic manifestation characterized by severe cholestasis developed during the recovery phase in patients with the critical form of COVID-19, named “post-COVID-19 cholangiopathy”[12]. Several mechanisms inducing the cholangiocyte damage have been proposed by researchers and will be briefly described below.
SARS-CoV-2 may infect the intestine, the liver, the kidneys and the brain cells. This variety of clinical manifestations is detectable not only during the acute phase of the disease but also in the recovery process[13]. The entry of the virus into the cell is preceded by the interaction of the pathogen with the ACE-2 receptor. The interaction is widely distributed in all the human tissues and easily observable in the liver and in the biliary tract[14,15]. In particular, increased mitotic activity of swollen hepatocytes, an enhanced rate of apoptosis visible in cells obtained from liver biopsies of COVID-19 patients as well as the abundance of the ACE-2 receptor in the different types of liver cells, provide evidence that SARS-CoV-2 exhibits a substantial affinity for these hepatic cells[16]. Therefore, cholangiocytes, hepatocytes and bile duct cells represent an ideal reservoir for SARS-CoV-2[17].
A high expression of ACE-2 receptors and transmembrane serine protease 2 (TMPRSS2) has been reported in enteric neurons and in glial cells of the small and large intestines[18]. A recent study has shown that this enteric nervous system allows SARS-CoV-2 to reach the biliary tract of the liver by exploiting the well-known gut-liver axis[17]. ACE-2 receptors in cholangiocytes support a retrograde mode of liver damage after the virus has entered the biliary tree cells[19,20]. Liver biopsies confirm the presence of viral RNA in the liver tissues. Atypical signs of hepatocyte damage, such as cellular apoptosis along with swelling, acidophilic bodies and lobular inflammation, have been observed too, characterized by the mechanism of direct viral damage[21]. Some pathogenetic mechanisms have been correlated with tissue damage in these individuals, including ACE-2-mediated direct viral infection of hepatocytes. The virus could even infect cholangiocytes and dysregulate the functions of both the biliary tract cells and the entire hepatic gland, causing a direct liver injury[22-24] owing to the generation of organelles damage[10,22].
Acute and persistent lobular inflammatory damage may occur in the liver of patients with COVID-19. This process is characterized by: (1) Elevated levels of circulating proinflammatory cytokines/ chemokines and other mediators, eventually triggering a cytokine storm and inducing liver dysfunction, as observed in a series of viral infections[22,25-27]; (2) A close association between liver injury and inflammatory responses whilst in SARS-CoV-2 infection[27], as patients with COVID-19 may incur hepatocellular damage, ranging from mild injuries to liver failure; and (3) Hepatotoxicity of drugs[22].
SARS-CoV-2 virions have been isolated in the bronchoalveolar fluid, in the sputum and in the blood samples of patients with COVID-19. However, recent evidence suggests the gastrointestinal tract represents a potential route of infection and transmission of this pathogen. Viable viral particles and RNA of SARS-CoV-2 have also been found in the feces of people suffering from COVID-19[28], meaning they may also represent a potential route of transmission. In synthesis, available studies show that: (1) It is possible a fecal-oral route of SARS-CoV-2 transmission in the gastrointestinal system and the virus replicates in the mucosa of the intestinal epithelial cells[29]; (2) A high expression of receptors and candidate coreceptors/auxiliary proteins can be identified in the gastrointestinal tract with an affinity for SARS-CoV-2; (3) An elevated expression of TMPRSS2 of the host is detectable in the cells of the gastrointestinal tract; (4) Following COVID-19 infection, the stool test for viral SARS-CoV-2 RNA gives a positive result for a considerable time in approximately 64% of patients with negative nasopharyngeal swab[30,31]; and (5) SARS-CoV-2 mRNA and its intracellular nucleocapsid protein can be observed in gastric, duodenal and rectal epithelia[32].
In order to pursue the objective of this research, we performed an extensive bibliographic search of the published works available in the literature concerning post-COVID-19 cholangiopathy. Then we conducted a systematic review of this topic.
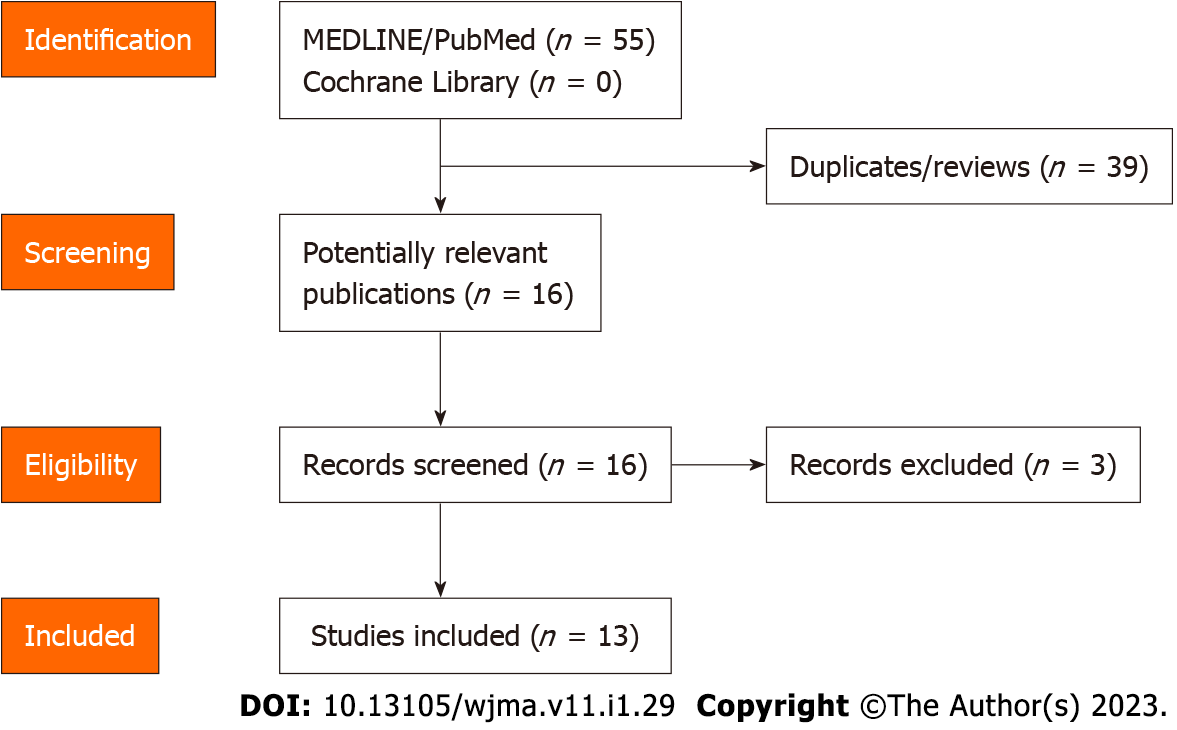
A systematic computer-based search of articles available in the literature was conducted through two electronic databases (MEDLINE/PubMed and Cochrane Library) with the aim of identifying relevant papers about post-COVID-19 cholangiopathy published between January 1, 2020 and August 22, 2022. Articles in all languages were considered. The MeSH terms and the keywords used were: “cholangiopathy,” “COVID-19,” “post-COVID-19 cholangiopathy” and “SARS-CoV-2.” The authors used the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to it[33]. Two of the authors (de Biase D and Gallo CG) independently and in parallel carried out the literature search and identified the relevant articles based on the title and/or the abstract. The inclusion criteria considered in our analysis were: retrospective and prospective studies, cohort studies, case series and case reports. Two additional authors (Hong W and Grottesi A) independently extracted and tabulated all the relevant data from the selected studies. Fiorino S controlled the accuracy of the data extracted. When an inconsistency of the results emerged between the selected papers, a consensus among all the authors was required. To avoid possible duplicates, we looked for the first author’s name, the place and the period of the enrollment of the subjects. The identified studies are depicted in Figure 1. In addition, we conducted a relevant search to supplement latest research results by Reference Citation Analysis (https://www.referencecitationanalysis.com/) when revising the manuscript.
The heterogeneity of data as well as the small size limited the ability to perform a comparative statistical analysis or a meta-analysis. Only a descriptive analysis with percentages has been carried out, not using any specific software.
A total of 16 articles have been identified describing patients with post-COVID-19 cholangiopathy. Three were excluded for the following reasons: two papers described the cholestasis caused by intravenous ketamine used for the sedation of patients with acute respiratory distress syndrome (ARDS)[[34,35]; and the third concerned a retrospective study of 72 cholestatic patients observed as early as 28 d after admission[36]. The included studies are summarized in Table 1.
| Ref. | SARS-CoV-2 patient age, sex | Known liver diseases | ICU, mechanical ventilation | Time peak of ALP since COVID-19 diagnosis | Liver biopsy (time) | ERCP (time) | LT |
| Edwards et al[37], 2020 | 59 yr, male | No | Yes | 79 d | Planned | Sludge clearance (2 procedures) | Planned for LT |
| Roth et al[11], 2021 | 38 yr, male | No | Yes | 139 d | Yes (day 151) | Sludge extraction (day 180) | No |
| 25 yr, male | No | Yes | 103 d | Yes (day 96) | Sludge and stones extraction (day 89 and 100) | No | |
| 40 yr, female | No | Yes | 172 d | Yes (day 178) | Not performed | No | |
| Durazo et al[38], 2021 | 47 yr, male | No | Yes | 81 d | Yes | Stone extraction and findings of SSC (day 73 and 81) | Yes |
| Lee et al[39], 2021 | 64 yr, male | No | Yes | 60 d | No | Stone, extraction, insertion of 8.5 Fr biliary stent and findings of SSC (day 52 and day 150) | Yes |
| Faruqui et al[40], 2021 | 12 patients, mean age 58 yr (11 males, 1 females) | No | Yes | 118 d | 4 patients | 4 patients | 1 patient and 1 planned for LT, 2 patients declined |
| Rojas et al[41], 2021 | 29 yr, female | No | Yes | 69 d | Yes | Negative | No |
| Bütikofer et al[42], 2021 | 11 patients with mild cholestasis (9 males, 2 females), 59 yr (range: 52-70) | No | Yes | 1.7 d (range: 1.2-2.0 d) | 4/9 patients (44%) | Not performed | 1 planned for LT |
| 9 patients with severe cholestasis (7 males, 2 females), 59 yr (range: 53-68) | No | Yes | 5.4 d (range: 2.5-7.4 d) | No | Not performed | No | |
| Franzini et al[43], 2022 | 65 yr, male | No | Yes | 63 d | No | Biliary casts removal | No |
| Santisteban Arenas et al[44], 2002 | 55 yr, male | No | Yes | 74 d | Yes (in three patients) | Stones extraction in 1 patient | No |
| 54 yr, male | No | Yes | 34 d | No | |||
| 62 yr, male | No | Yes | 88 d | No | |||
| 56 yr, female | No | Yes | 39 d | No | |||
| 73 yr, female | No | Yes | 82 d | No | |||
| 34 yr, male | Hepatic hemangiomas | Yes | 95 d | No | |||
| Ludwig et al[45], 2022 | 69 yr, male | Not known | Not known | Not known | Not known | Diffuse beading and stricturing of the intrahepatic bile ducts | Yes |
| Rela et al[46], 2022 | 50 yr, male | No | Yes | 42 d (serum bilirubin) | Yes | Not performed | Yes |
| Kulkarni et al[47], 2022 | 8 patients unvaccinated, 59 yr (range: 24-67), all males | Fatty liver (2 patients) | 7 patients (87.5%) | 571.5 d (range: 368-1058 d) | 5 patients | Not performed | 2 patients and 4 patients planned for LT |
| 7 patients vaccinated, 52 yr (range: 29-67), 5 males and 2 females | Fatty liver (4 patients) | 4 patients (57.1%) | 312 d (range: 239-517 d) | 2 patients | Not performed | No | |
| Roda et al[48], 2022 | 63 yr with bilateral lung transplant, male | No | Yes | 90 d | Cholangiopathy confirmed post-mortem | Not performed | No |
Taking into account the descriptive analysis of these 13 studies[11,37-48], the following data have been obtained: (1) 64 patients were examined, with a prevalence (82.8%) of males (53 males vs 11 females); (2) The average peak of ALP values was 75.5 d; (3) A liver biopsy was performed in 24 of the 64 patients (37.5%); (4) A total of 17 endoscopic retrograde cholangiopancreatography (ERCPs) were carried out, mainly to extract sludge and stones. During an examination, cholangioscopy was used to directly view the stenosed intrahepatic segment[43]; and (5) 6 patients received a liver transplant, while 7 patients have been scheduled for surgery. Two patients refused liver transplant. A total of 15 patients (23.4%) were eligible for a liver transplant.
Secondary sclerosing cholangitis in critically ill patients is a rare cholestatic condition encountered in patients developing sepsis or ARDS during a prolonged stay in the intensive care unit. This pathology rapidly induces cirrhosis, leading to liver failure. Its prognosis is poor, and the only option consists of a liver transplant. Some risk factors for post-COVID-19 cholangiopathy have been identified: mechanical ventilation, prone position and excess intraperitoneal fat[49]. Its pathogenesis is complex and is suggestive of a damage of ischemic origin that may involve the biliary tract until its stenosis and at the end a subsequent over infection caused by multidrug-resistant bacteria[49].
Roth et al[11] first noticed that the histological characteristics were similar to secondary sclerosing cholangitis in critically ill patients occurring in their patients, with severe damage to cholangiocytes. The injury of the cells has been characterized by a marked cytoplasmic vacuolization and by intrahepatic microangiopathy. This recognized pattern highly suggests a direct liver damage induced by SARS-CoV-2[11]. These findings have been the very first observations of secondary sclerosing cholangitis post-COVID-19. Hence, the authors suggested that post-infectious cholestasis could be due to an overlap of secondary sclerosing cholangitis in critically ill patients. This assumption is supported by a higher elevation of serum ALP levels registered in correlation with direct hepatic damage[11].
In a recent prospective cohort study, 461 patients with COVID-19 underwent liver function tests both during hospitalization and at 1, 3, 6 and 12 mo after their discharge[50]. The results showed that they markedly improved over time, with only 13.2% of tests altered at 12 mo compared to 25.1% in the 1st month[50]. Unfortunately, this study considered only GGT levels as a cholestasis index, with corresponding median values of 27 U/L (range: 18-40 U/L) in the 1st month of follow-up and 20 U/L (range: 13-29) after 1 year, without having tested and serum bilirubin levels[50].
In these subjects, the presence of a persistent cholestatic condition combined with jaundice requires diagnostic radiological integration. An intravenous contrast computed tomography scan of the abdomen may show both dilation of the intrahepatic bile ducts and of the common bile duct with hyperpotentiation of their walls[51]. A magnetic resonance cholangiopancreatography can provide other additional details, including the presence of diffuse periductal edema[52]. Finally, an invasive and therapeutic examination (ERCP), as we have observed in the works listed in Table 1, can show tortuosity of intrahepatic bile ducts[53].
The drugs used for the treatment of this infection include antivirals, antibiotics, antipyretics and immune modulators that often provoke transient hepatotoxicity[54,55]. With specific regard to its medical therapy, in most examined works it is reported that drugs such as ursodeoxycholic acid and obeticholic acid have been used, with the aim of not resolving the disease but only slowing down the liver damage produced by the accumulation of bile acids that were not excreted[56].
We are of the opinion that post-COVID-19 cholangiopathy represents a topic of interest that could entail future developments. Unfortunately, the low number of available studies and the small cases of enrolled patients constitute a current limit to our evaluation. In the near future, further investigations focused on this new emerging pathology based on a greater sample of subjects should be undertaken in order to better identify the best treatment.
Liver involvement during SARS-CoV-2 infection is mild and transient, as reported in the literature. Unfortunately, some cases of severe liver damage can occur, leading to the failure of the organ. According to the data emerged by reviewing the previous works, it can be asserted that post-COVID-19 cholangiopathy may represent a clinicopathological condition needing strict control owing to the high risk of developing progressive liver damage that might need a transplant. This research is quite innovative and shows interesting results, but because of its recent discoveries it meets some limitations, such as the low number of published studies and patients enrolled. Further investigations including a larger sample size could help in a better comprehension of the pathogenesis and of the development of this disease, preventing or at least mitigating its clinical course and improving its treatment.
Post-coronavirus disease 2019 (COVID-19) cholangiopathy is a recently identified clinical entity that develops during the recovery phase from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Early recognition of this complication is critical to ensure prompt and adequate management, which could affect the prognosis of these patients.
The main objectives of this review were to identify the available data contained in the studies accessible from the literature concerning post-COVID-19 cholangiopathy.
We have searched within two electronic databases (PubMed and the Cochrane Library) works on this topic, published between January 1, 2020 to August 22, 2022, using MeSH terms and free-language keywords: cholangiopathy; COVID-19; post-COVID-19 cholangiopathy; SARS-CoV-2.
Thirteen studies were included in this descriptive review, which included 64 patients suffering from this condition.
This review analyzed the possible causes and the clinical course of post-COVID-19 cholangiopathy, aiming to understand both its possible causes and its consequent clinical evolution.
Cholangiopathy is a medium-to-long-term complication of this virus, in which biliary damage is generally progressive up to liver failure. Researchers should focus on both early recognition and timely treatment of this complication.
The authors thank Dr. de Vito S, Italian Medicines Agency, Rome, Italy for English revision of the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia; Shariati MBH, Iran S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med. 2020;35:1545-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 809] [Cited by in F6Publishing: 671] [Article Influence: 167.8] [Reference Citation Analysis (0)] |
| 2. | Zippi M, Fiorino S, Occhigrossi G, Hong W. Hypertransaminasemia in the course of infection with SARS-CoV-2: Incidence and pathogenetic hypothesis. World J Clin Cases. 2020;8:1385-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Zippi M, Hong W, Traversa G, Maccioni F, De Biase D, Gallo C, Fiorino S. Involvement of the exocrine pancreas during COVID-19 infection and possible pathogenetic hypothesis: a concise review. Infez Med. 2020;28:507-515. [PubMed] [Cited in This Article: ] |
| 4. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3643] [Cited by in F6Publishing: 3949] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 5. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 278] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 6. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 315] [Article Influence: 78.8] [Reference Citation Analysis (1)] |
| 7. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5228] [Cited by in F6Publishing: 5537] [Article Influence: 1384.3] [Reference Citation Analysis (2)] |
| 8. | Kariyawasam JC, Jayarajah U, Abeysuriya V, Riza R, Seneviratne SL. Involvement of the Liver in COVID-19: A Systematic Review. Am J Trop Med Hyg. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 10. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1184] [Cited by in F6Publishing: 1223] [Article Influence: 305.8] [Reference Citation Analysis (4)] |
| 11. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 12. | Bethineedi LD, Suvvari TK. Post COVID-19 cholangiopathy - A deep dive. Dig Liver Dis. 2021;53:1235-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15248] [Cited by in F6Publishing: 13197] [Article Influence: 3299.3] [Reference Citation Analysis (0)] |
| 14. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 692] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 15. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1286] [Cited by in F6Publishing: 1444] [Article Influence: 361.0] [Reference Citation Analysis (0)] |
| 16. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 311] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 17. | Fiorino S, Tateo F, Biase D, Gallo CG, Orlandi PE, Corazza I, Budriesi R, Micucci M, Visani M, Loggi E, Hong W, Pica R, Lari F, Zippi M. SARS-CoV-2: lessons from both the history of medicine and from the biological behavior of other well-known viruses. Future Microbiol. 2021;16:1105-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Deffner F, Scharr M, Klingenstein S, Klingenstein M, Milazzo A, Scherer S, Wagner A, Hirt B, Mack AF, Neckel PH. Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV2. Front Neuroanat. 2020;14:596439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 818] [Cited by in F6Publishing: 834] [Article Influence: 208.5] [Reference Citation Analysis (0)] |
| 20. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12421] [Article Influence: 3105.3] [Reference Citation Analysis (1)] |
| 21. | Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172:577-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4286] [Cited by in F6Publishing: 3266] [Article Influence: 816.5] [Reference Citation Analysis (1)] |
| 22. | Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 429] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 23. | Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1854] [Cited by in F6Publishing: 1941] [Article Influence: 485.3] [Reference Citation Analysis (0)] |
| 24. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1137] [Article Influence: 284.3] [Reference Citation Analysis (0)] |
| 25. | Ali N, Hossain K. Liver injury in severe COVID-19 infection: current insights and challenges. Expert Rev Gastroenterol Hepatol. 2020;14:879-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1894] [Cited by in F6Publishing: 2588] [Article Influence: 647.0] [Reference Citation Analysis (0)] |
| 29. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4227] [Cited by in F6Publishing: 4268] [Article Influence: 1067.0] [Reference Citation Analysis (0)] |
| 30. | Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, Yang Y, Liu B, Wang W, Wei C, Yang J, Ye G, Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 535] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 31. | Chen C, Gao G, Xu Y, Pu L, Wang Q, Wang L, Wang W, Song Y, Chen M, Yu F, Yang S, Tang Y, Zhao L, Wang H, Wang Y, Zeng H, Zhang F. SARS-CoV-2-Positive Sputum and Feces After Conversion of Pharyngeal Samples in Patients With COVID-19. Ann Intern Med. 2020;172:832-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 32. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1905] [Article Influence: 476.3] [Reference Citation Analysis (1)] |
| 33. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47017] [Cited by in F6Publishing: 43435] [Article Influence: 2895.7] [Reference Citation Analysis (0)] |
| 34. | Keta-Cov research group. Electronic address: vincent.mallet@aphp.fr.; Keta-Cov research group. Intravenous ketamine and progressive cholangiopathy in COVID-19 patients. J Hepatol. 2021;74:1243-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Wendel-Garcia PD, Erlebach R, Hofmaenner DA, Camen G, Schuepbach RA, Jüngst C, Müllhaupt B, Bartussek J, Buehler PK, Andermatt R, David S. Long-term ketamine infusion-induced cholestatic liver injury in COVID-19-associated acute respiratory distress syndrome. Crit Care. 2022;26:148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Da BL, Suchman K, Roth N, Rizvi A, Vincent M, Trindade AJ, Bernstein D, Satapathy SK; Northwell COVID-19 Research Consortium. Cholestatic liver injury in COVID-19 is a rare and distinct entity and is associated with increased mortality. J Intern Med. 2021;290:470-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Durazo FA, Nicholas AA, Mahaffey JJ, Sova S, Evans JJ, Trivella JP, Loy V, Kim J, Zimmerman MA, Hong JC. Post-Covid-19 Cholangiopathy-A New Indication for Liver Transplantation: A Case Report. Transplant Proc. 2021;53:1132-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 39. | Lee A, Wein AN, Doyle MBM, Chapman WC. Liver transplantation for post-COVID-19 sclerosing cholangitis. BMJ Case Rep. 2021;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 41. | Rojas M, Rodríguez Y, Zapata E, Hernández JC, Anaya JM. Cholangiopathy as part of post-COVID syndrome. J Transl Autoimmun. 2021;4:100116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Bütikofer S, Lenggenhager D, Wendel Garcia PD, Maggio EM, Haberecker M, Reiner CS, Brüllmann G, Buehler PK, Gubler C, Müllhaupt B, Jüngst C, Morell B. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404-2417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 43. | Franzini TAP, Guedes MMF, Rocha HLOG, Fleury CA, Bestetti AM, Moura EGH. Cholangioscopy in a post-COVID-19 cholangiopathy patient. Arq Gastroenterol. 2022;59:321-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Santisteban Arenas MT, Osorio Castrillón LM, Guevara Casallas LG, Niño Ramírez SF. [Post-COVID-19 severe cholangiopathy: report of 6 cases]. Rev Gastroenterol Peru. 2022;42:53-57. [PubMed] [Cited in This Article: ] |
| 45. | Ludwig DR, Anderson MA, Itani M, Sharbidre KG, Lalwani N, Paspulati RM. Secondary sclerosing cholangitis: mimics of primary sclerosing cholangitis. Abdom Radiol (NY). 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Rela M, Rajakannu M, Veerankutty FH, Vij M, Rammohan A. First report of auxiliary liver transplantation for severe cholangiopathy after SARS-CoV-2 respiratory infection. Am J Transplant. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Kulkarni AV, Khlegi A, Sekaran A, Reddy R, Sharma M, Tirumalle S, Gora BA, Somireddy A, Reddy J, Menon B, Reddy DN, Rao NP. Post COVID-19 cholestasis: a case series and review of literature. J Clin Exp Hepatol. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Roda S, Ricciardi A, Maria Di Matteo A, Zecca M, Morbini P, Vecchia M, Chiara Pieri T, Giordani P, Tavano A, Bruno R. Post-acute coronavirus disease 2019 (COVID 19) syndrome: HLH and cholangiopathy in a lung transplant recipient. Clin Infect Pract. 2022;15:100144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Kirchner GI, Rümmele P. Update on Sclerosing Cholangitis in Critically Ill Patients. Viszeralmedizin. 2015;31:178-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Liao X, Li D, Ma Z, Zhang L, Zheng B, Li Z, Li G, Liu L, Zhang Z. 12-Month Post-Discharge Liver Function Test Abnormalities Among Patients With COVID-19: A Single-Center Prospective Cohort Study. Front Cell Infect Microbiol. 2022;12:864933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Aldossary B, Hassan A, Moussa M, Alsaif HS, Alfaraj D. Fulminant hepatic failure in a patient testing re-positive for SARS-CoV-2: a case report. Int J Emerg Med. 2021;14:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Ghafoor S, Germann M, Jüngst C, Müllhaupt B, Reiner CS, Stocker D. Imaging features of COVID-19-associated secondary sclerosing cholangitis on magnetic resonance cholangiopancreatography: a retrospective analysis. Insights Imaging. 2022;13:128. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 53. | Trejo-Paredes C, Mohammed TJ, Salmon A. COVID-19-induced liver injury: a clinical distraction? Chest. 2020;158:A968. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 203] [Article Influence: 67.7] [Reference Citation Analysis (2)] |
| 55. | Heucke N, Keitel V. COVID-19-associated cholangiopathy: What is left after the virus has gone? Hepatology. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Caballero-Alvarado J, Corvera CZ, Bacilio BM, Caballero CR, Lozano-Peralta K. Post covid cholangiopathy: A narrative review. Gastroenterol Hepatol. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 10] [Reference Citation Analysis (0)] |