Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1726
Peer-review started: January 31, 2019
First decision: April 18, 2019
Revised: May 7, 2019
Accepted: May 23, 2019
Article in press: May 23, 2019
Published online: July 6, 2019
Appendiceal mucinous neoplasm (AMN) is extremely rare. Since the disease does not manifest a characteristic profile of clinical symptoms, it is easy to misdiagnose and still difficult to diagnose without operation. Here, we report a case of low-grade AMN (LAMN) and summarize its clinical features, diagnosis, and treatment.
A 63-year-old postmenopausal woman presented with a history of right lower abdominal mass. The patient underwent laparotomy, which showed an appendiceal mucocele originating from the apex of the appendix, and a simple appendectomy was performed. The subsequent histological assessment identified an LAMN with no lymph node involvement and negative surgical margin. The patient received six cycles of chemotherapy after surgery, and to date, more than a year after the surgery, the patient remains in good health.
A unified, standardized, detailed, and accurate pathological diagnosis is needed for LAMN, to facilitate selection of an appropriate surgical plan. In addition, the surgeon should record the details of the tumors in the surgical records in order to facilitate follow-up after surgery.
Core tip: Appendiceal mucinous neoplasms (AMNs) are rare tumors. Only a few cases have been reported and most patients have no typical clinical manifestations. As such, diagnosis and treatment are a clinical challenge; even the terminology of AMN grading and staging has been controversial and is not standardized yet. Malignant AMNs have a poor curative effect and no treatment standard has been established. Here, we report a female patient with AMN who has survived throughout follow-up, now at 1 year post-surgery. A review of the latest literature is also provided to improve the overall awareness and understanding of this disease.
- Citation: Yang JM, Zhang WH, Yang DD, Jiang H, Yu L, Gao F. Giant low-grade appendiceal mucinous neoplasm: A case report. World J Clin Cases 2019; 7(13): 1726-1731
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1726.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1726
Appendiceal mucinous neoplasms (AMNs) are a rare group of tumors. The reported incidence is 0.2%-0.4% among all patients who have undergone appendectomy, and they were found most frequently in patients aged 50-60 years[1,2]. This disease is extremely easy to misdiagnose, and missed diagnosis is common. Most cases do not manifest any typical clinical symptoms, and ANM is often found incidentally in physical examination or other operations[3]. Only a small number of patients present with the nonspecific symptoms of chronic right lower abdominal pain or mass.
Correct preoperative diagnosis is helpful to prevent rupture of the tumor during operation, which can lead to iatrogenic implantation and the formation of pseu-domyxoma peritonei (PMP)[4]. Herein, we report a case of low-grade (L)AMN that was diagnosed by surgical and histological findings, and treated at our hospital. The latest literature is also reviewed, with the objective of improving awareness and understanding of this disease.
A 63-year-old postmenopausal woman presented with a history of mass and pain in the lower right abdomen.
More than half a year prior to presentation, the patient developed a noticeable mass in her right lower abdomen that was accompanied by mild pain and discomfort, which did not prompt serious concern at that time. The mass gradually increased in size and the symptoms escalated in intensity (i.e., abdominal pain) and type (i.e., distension). She went to the local hospital first, but then came to our hospital seeking further diagnosis and treatment.
The patient had no history of past illness.
Mild tenderness was found in the right lower abdomen and a mass of about 15 cm × 8 cm was detected by palpation.
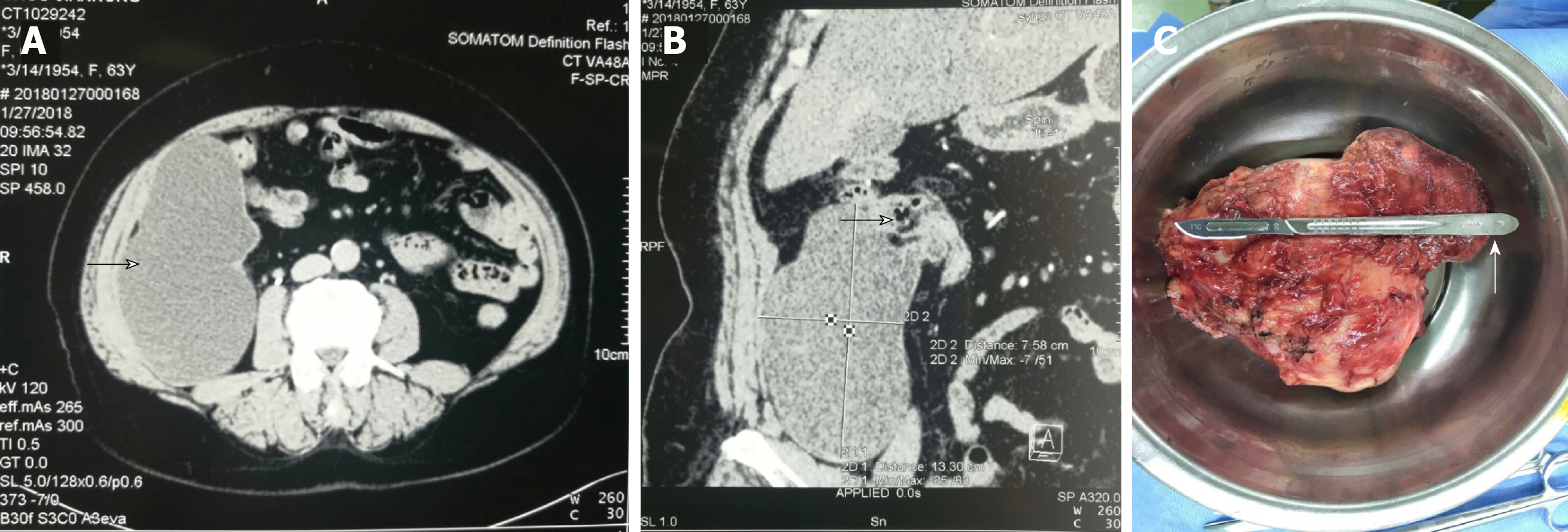
Ultrasonography revealed a huge cystic mass of 7 cm × 13 cm in the right ileocecal-appendix region, which had a blurred border with the surrounding tissue. Plain and enhanced computed tomography (CT) examinations showed cystic low-density lesions with partial calcification in the appendix area of the right iliac fossa. The size of the lesions was about 7.6 cm × 13.3 cm, and the CT value was 17 HU. No obvious enhancement was found in any of the stages after enhancement. No obvious enlarged lymph nodes were found in the abdominal cavity and retroperitoneum.
We began to suspect an appendiceal abscess or cystic tumor, as well as the possibility of mucocele of the appendix (Figure 1). As such, testing for serum tumor markers was carried out. All markers were within normal ranges, except for the carcinoembryonic antigen (commonly known as CEA; 8.63 ng/mL, normal range: < 5 ng/mL).
Exploratory laparotomy was performed. A huge cystic mass measuring about 8 cm × 10 cm × 15 cm in size was found in the right lower abdomen. The mass originated from the apex of the appendix, adhered to the right abdominal wall and retro-peritoneum, and had the greatest amount of fixation to the right iliac bone.
Pathologists Ying Jiang (Pathology Department, Second Affiliated Hospital of Harbin Medical University) and Xiao-Yu Yu (Pathology Department, Third Affiliated Hospital of Harbin Medical University) performed histological assessment of the resected mass and surrounding tissues. Immunohistochemical detection was per-formed. They assisted in the selection of pathological images.
A diagnosis of LAMN was made. Immunohistochemical staining for CK was positive (Figure 2).
The postsurgical recovery was uneventful. The patient was discharged to home on the 6th day and was referred for six cycles of chemotherapy (capecitabine plus oxa-liplatin, known as the XELOX regimen), which was carried out at the local hospital.
The follow-up routine was designed to involve testing of tumor markers [CEA and carbohydrate antigen (commonly known as CA19-9)] and CT scans every 6 mo. To date, the patient has completed 18 months of the follow-up and remains in good health, with no sign of recurrence.
The first published description of an appendiceal mucocele was provided by Rokitansky[5], who characterized the novel finding as an abnormal accumulation of mucus leading to an expansion of the appendiceal cavity. Since then, there has been a great deal of controversy about the proper and accurate terminology of AMN classification and staging, leaving pathologists and surgeons with a greater clinical challenge in assessing and managing the disease[6]. At present, the most widely used system is the 2010 4th edition of the World Health Organization digestive system tumor classification[7] which classifies the tumors into five groups: Mucinous adenoma /cystadenoma (MN); LAMN; low-grade peritoneal pseudomyxoma originating from the appendix (referred to as PMP-L); mucinous adenocarcinoma of the appendix; and high-grade peritoneal pseudomyxoma originating from the appendix (referred to as PMP-H).
The main auxiliary diagnostic methods today are abdominal ultrasound and abdominal CT, with colonoscopy. Ultrasound can show a mass with small echo spots and/or a concentric echo layer (known as the “onion skin”), both of which have been proposed as ANM-specific alterations[8]. Yet another study has shown that when the diameter of the ultrasound-visualized appendix exceeds 15 mm, the diagnosis for AMN has a sensitivity of 83% and a specificity of 92%[2]. CT, however, is considered to be the most informative imaging method[9], as it can evaluate the relationship between the formation of peripheral organs, which may make diagnosis easier.
When CT manifestations are cystic structures closely related to the cecum with round or long tubular shape, thin cyst wall, and smooth outline, the possibility of appendiceal mucinous adenocarcinoma should be considered; irregular cyst wall and soft tissue thickening may be also be indicative. The finding of appendix cavity diameter exceeding 13 mm prompts high suspicion of AMN.
An important indication found during colonoscopy is the so-called “volcano sign” - a visible raised zone in the cecum, with an appendicular orificium located in its center[10]. Electronic colonoscopy performance is important in the diagnostic aspect, as it allows for exclusion of synchronous or metachronous colon tumors. We believe that a comprehensive examination, involving imaging and surgical investigations, will help to improve the diagnostic rate, but the final diagnosis still depends on patho-logical examination of resected tissues.
Surgery is the only treatment with curative potential. During the operation, caution should be taken to avoid rupture and spread of the mucus or epithelial cells to the abdominal cavity and consequent formation of a PMP, which can significantly affect the prognosis of patients due to the high recurrence rate[11]. Currently, many teams consider the best strategy for obtaining better long-term results after treating PMP to be initiation of appropriate treatment of the peritoneal disease as early as possible; the option of no treatment would simply equate to waiting for the patient to re-present in a more advanced stage and weaker status. The peritoneal cancer index (commonly known as the PCI and used to measure the degree of peritoneal disease) is an independent factor predictive of postoperative morbidity and poor survival of AMP cases[12].
Laparoscopic surgical approach provides better evaluation of the entire abdominal cavity. Unfortunately, it carries the increased risk of rupture of the mucocele and provocation of a PMP[3]. We agree with the scholars who believe that laparoscopic surgery is a safe and reliable treatment for appendiceal mucocele[13], according to our experience. It is important to remember, however, that if the cyst is larger or the lesion is more adherent, the risk of tumor rupture and injury will increase, and laparotomy should be performed decisively. In recent years, more reports have supported use of the aggressive strategy developed by Dhage-Ivatury and Sugarbaker[14], which involves peritoneal and visceral resection and is also known as cytopenic surgery and hyperthermic intraperitoneal chemotherapy (CRS + HIPEC).
In any case, at the time of first operation, the appendix and the adipose tissue around its mesentery should be removed. If any liquid or mucus is found, cytological examination must be performed[15]. Some scholars believe that if negative results are obtained from the interoperative frozen sections of lymph nodes in and along the appendiceal artery, then right hemicolectomy can be avoided. In addition, a negative incision margin for the appendix can be obtained by simple appendectomy, which can also preserve function of the ascending colon and the ileocecal valve[16]. If a negative margin is not obtained, an ileocecal excision can be performed. However, a recent study has shown that for LAMN patients whose tumors were confined to the appendix and tumor markers were at normal levels, appendectomy alone (+/-) could not predict the recurrence and peritoneal dissemination of the disease, but noted that in general, even without further surgery, the appendectomy alone was sufficient[17]. In our case, the tumor was located at the apex of the appendix with a negative incision margin and slightly increased CEA level before operation. After consulting with the patient’s family members during the operation, it was decided to perform only the appendectomy and then administer regular chemotherapy after the operation.
When emergency surgery fails to provide results from intraoperative frozen section analysis of the lymph nodes, a right hemicolectomy should not be performed because malignant tumors account for only 10%-20% of mucoceles[15]. The reported incidence of PMP is 65% in MN cases with perforation and 17% in MN cases without perforation; therefore, for patients without perforation, the possibility of PMP formation cannot be completely ruled out[18]. For patients with LAMN, prophylactic CRS + HIPEC treatment can achieve the greatest survival benefit, but there is an unknown risk of overtreatment. However, systemic chemotherapy before CRS + HIPEC treatment has been shown to significantly improve the prognosis of patients with peritoneal mucinous cancer[19].
When patients have both ovarian tumors and AMN, molecular analysis has revealed that the appendix is the primary source and the ovarian tumors are se-condary. Therefore, in order to avoid missed diagnosis of early implanted lesions, PMP patients who are postmenopausal should have a bilateral ovariectomy per-formed at the same time as the appendectomy[20].
Treatment strategies for patients with AMN perforation should be standardized. The following plan for management of appendix epithelial tumor perforation cases has been put forward by Sugarbaker et al[21]: (1) For AMN with histologic mani-festations of disseminated peritoneal adenomucinosis, appendectomy with CRS + HIPEC is recommended; (2) For patients with histological results showing well or moderately differentiated PMCA, the same recommendations as in (1) are made; and (3) Finally, for patients with histological results showing poorly differentiated PMCA or intestinal (non-mucinous) tumors, right hemicolectomy with regional lymph node dissection is required. In the absence of right hemicolectomy, negative findings in appendiceal lymph node samples indicate no lymph node metastasis. Using this sentinel lymph node sampling method, the implicit lymph nodes in the ileocecal colon group are retained, which is conducive to follow-up review and secondary surgical evaluation.
AMN is a rare tumor, and it is necessary to enhance the awareness and understanding of this disease. Its prognosis depends on the overall nature and type of the tumor. Pathologists should make a unified, standardized, detailed, and accurate pathological diagnosis scheme to facilitate selection of the most appropriate surgical treatment plan. Furthermore, the treating surgeons should provide detailed descriptions of the size and location of the tumor, location of abdominal mucus, and of any perforation, in the surgical records for easy follow-up. During the operation for removal of AMNs, the aim must be to ensure complete excision by means of delicate and gentle operative maneuvers in order to avoid mucus spillover after cyst wall rupture and its con-sequent dispersion into the abdominal cavity to form PMPs. Postoperative follow-up should be carried out regularly. CRS and HIPEC are standard treatments for peritoneal metastasis of LAMN. Unfortunately, this technique has not been developed at our center.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lambrecht NW, Pekgoz M, Yakoot M S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Zagrodnik DF 2nd, Rose DM. Mucinous cystadenoma of the appendix: diagnosis, surgical management, and follow-up. Curr Surg. 2003;60:341-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Karakaya K, Barut F, Emre AU, Ucan HB, Cakmak GK, Irkorucu O, Tascilar O, Ustundag Y, Comert M. Appendiceal mucocele: case reports and review of current literature. World J Gastroenterol. 2008;14:2280-2283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Hassan S, Dhebri A, Lin L, Haque M. Appendiceal mucocele: a missed diagnosis. BMJ Case Rep. 2013;2013:pii: bcr2012007983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Bennett GL, Tanpitukpongse TP, Macari M, Cho KC, Babb JS. CT diagnosis of mucocele of the appendix in patients with acute appendicitis. AJR Am J Roentgenol. 2009;192:W103-W110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Rokitansky C. A Manual of Pathological Anatomy, vol. 2, Blanchard Lea, Philadelphia, Pa, USA, 1855. . [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Misdraji J. Appendiceal mucinous neoplasms: controversial issues. Arch Pathol Lab Med. 2010;134:864-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Bosman FT; World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer 2010; 417 Available from: http://apps.who.int/bookorders/WHP/detart1.jsp?sesslan=1codlan=1codcol=70codcch=4003. [Cited in This Article: ] |
| 8. | Garg PK, Prasad D, Aggarwal S, Mohanty D, Jain BK. Acute intestinal obstruction: an unusual complication of mucocele of appendix. Eur Rev Med Pharmacol Sci. 2011;15:99-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Rymer B, Forsythe RO, Husada G. Mucocoele and mucinous tumours of the appendix: A review of the literature. Int J Surg. 2015;18:132-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Shiihara M, Ohki T, Yamamoto M. Preoperative Diagnosis and Surgical Approach of Appendiceal Mucinous Cystadenoma: Usefulness of Volcano Sign. Case Rep Gastroenterol. 2017;11:539-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Djuranovic SP, Spuran MM, Kovacevic NV, Ugljesic MB, Kecmanovic DM, Micev MT. Mucinous cystadenoma of the appendix associated with adenocarcinoma of the sigmoid colon and hepatocellular carcinoma of the liver: report of a case. World J Gastroenterol. 2006;12:1975-1977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, González-Moreno S, Van Der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449-2456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 687] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 13. | Park KJ, Choi HJ, Kim SH. Laparoscopic approach to mucocele of appendiceal mucinous cystadenoma: feasibility and short-term outcomes in 24 consecutive cases. Surg Endosc. 2015;29:3179-3183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Dhage-Ivatury S, Sugarbaker PH. Update on the surgical approach to mucocele of the appendix. J Am Coll Surg. 2006;202:680-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Sugarbaker PH. Epithelial appendiceal neoplasms. Cancer J. 2009;15:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | De Abreu Filho JG, De Lira EF. Mucocele of the appendix: appendectomy or colectomy? J Coloproctol. 2011;31:276-284. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Fournier K, Rafeeq S, Taggart M, Kanaby P, Ning J, Chen HC, Overman M, Raghav K, Eng C, Mansfield P, Royal R. Low-grade Appendiceal Mucinous Neoplasm of Uncertain Malignant Potential (LAMN-UMP): Prognostic Factors and Implications for Treatment and Follow-up. Ann Surg Oncol. 2017;24:187-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Honoré C, Caruso F, Dartigues P, Benhaim L, Chirica M, Goéré D, Elias D. Strategies for Preventing Pseudomyxoma Peritonei After Resection of a Mucinous Neoplasm of the Appendix. Anticancer Res. 2015;35:4943-4947. [PubMed] [Cited in This Article: ] |
| 19. | Milovanov V, Sardi A, Ledakis P, Aydin N, Nieroda C, Sittig M, Nunez M, Gushchin V. Systemic chemotherapy (SC) before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) in patients with peritoneal mucinous carcinomatosis of appendiceal origin (PMCA). Eur J Surg Oncol. 2015;41:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González-Moreno S, Taflampas P, Chapman S, Moran BJ; Peritoneal Surface Oncology Group International. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. 2016;40:14-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 414] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 21. | Sugarbaker PH. When and When Not to Perform a Right Colon Resection with Mucinous Appendiceal Neoplasms. Ann Surg Oncol. 2017;24:729-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |