Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.534
Peer-review started: November 10, 2022
First decision: November 26, 2022
Revised: December 22, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 26, 2023
Patients with cirrhosis have an increased risk of infection and differently from other complications, that over the years are improving in their outcomes, infections in cirrhotic patients are still a major cause of hospitalization and death (up to 50% in-hospital mortality). Infections by multidrug-resistant organisms (MDRO) have become a major challenge in the management of cirrhotic patients with significant prognostic and cost-related impact. About one third of cirrhotic patients with bacterial infections is infected with MDR bacteria and their prevalence has increased in recent years. MDR infections have a worse prognosis compared to infections by non-resistant bacteria because they are associated with lower rate of infection resolution. An adequate management of cirrhotic patients with infections caused by MDR bacteria depends on the knowledge of some epidemiological aspects, such as the type of infection (spontaneous bacterial peritonitis, pneumonia, urinary tract infection and spontaneous bacteremia), bacteriological profile of antibiotic resistance at each health care unit and site of infection acquisition (community acquired, healthcare associated or nosocomial). Furthermore, regional variations in the prevalence of MDR infections determine that the choice of empirical antibiotic therapy must be adapted to the local microbiological epidemiology. Antibiotic treatment is the most effective measure to treat infections caused by MDRO. Therefore, optimizing antibiotic prescribing is critical to effectively treat these infections. Identification of risk factors for multidrug resistance is essential to define the best antibiotic treatment strategy in each case and the choice of an effective empirical antibiotic therapy and its early administration is cardinal to reduce mortality. On the other hand, the supply of new agents to treat these infections is very limited. Thus, specific protocols that include preventive measures must be implemented in order to limit the negative impact of this severe complication in cirrhotic patients.
Core Tip: Infections by multidrug-resistant organisms (MDRO) have become a major challenge in the management of cirrhotic patients with significant prognostic and cost-related impact. This review presents the main epidemiological data, clinical impact, risk factors, and the best management of cirrhotic patients infected with MDR bacteria.
- Citation: Terra C, de Mattos ÂZ, Chagas MS, Torres A, Wiltgen D, Souza BM, Perez RM. Impact of multidrug resistance on the management of bacterial infections in cirrhosis. World J Clin Cases 2023; 11(3): 534-544
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/534.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.534
The rising prevalence of multidrug-resistant organisms (MDRO) (resistant at least to one agent in three or more antimicrobial categories), extensively drug-resistant organisms (a resistance profile that compromised at least one agent in all but two or fewer antimicrobial categories), and pan drug-resistant organisms (PDRO) (resistant to all known antimicrobial agents) represents a global threat to human health[1,2]. At same time, active agents against MDRO are limited despite an increase in the availability of novel antibiotics in recent years.
Reports from the United States and Europe estimate a death toll of 29-33 patients each year associated with antimicrobial resistant microorganisms, with a huge attributable healthcare cost[2-5]. The so called “ESKAPE” pathogens [Enterococcus faecium, Staphylococcus aureus (S. aureus), Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae species] are especially worrisome: The acquisition of antimicrobial resistance genes, in association to the classical mechanisms of antimicrobial resistance (inactivation or alteration of the antimicrobial molecule, bacterial target site modifications, reduced antibiotic penetration/accumulation, and the formation of bacterial biofilms), make ESKAPE pathogens challenging to surveillance and subsequent infections difficult to treat. The SKAPE pathogens have developed resistance against almost all antibiotics used in the clinical setting (oxazolidinones, lipopeptides, macrolides, fluoroquinolones, B-lactams, and also to antibiotics that are considered "the last line of defense”, like carbapenems, glycopeptides and polymyxins). Face to the increased burden of disease and death rates due to treatment failure, the Word Health Organization has designated the SKAPE group as “priority organisms” to focus and guide research and development related to new antibiotics[1].
Over the last years cirrhosis complications are improving their outcomes with new strategies and technologies. However, infections in cirrhotic patients are still a major cause of hospitalization and death (up to 50% in-hospital mortality)[6]. Although the reasons for these imbalanced morbidity and mortality are not totally elucidated, MDR infections may play an important role. In a recent multicentric study that assessed the epidemiology of bacterial infections in hospitalized cirrhotic patients, the overall prevalence of MDRO was 34%[7]. Another report concerning critically ill patients with decompensated cirrhosis points to 46% of isolates being MDROs at admissions. Meanwhile, MDRO isolates responsible for infection during intensive care unit stay were at 60%[8]. Different studies confirm the ominous prognosis of cirrhotic patients with infections by MDRO[9,10] (Table 1) Antimicrobial standard prescription for infection in liver cirrhosis doesn’t routinely comprehend the MDRO spectrum. Cirrhosis itself increases the risk for sepsis and septic shock and when associated with an inappropriate choice of empirical antimicrobial treatment may be determinant of a worst outcome.
| Ref. | Study design | n | MDR infection (%) | Mortality (%) | Comments |
| Piano et al[7] | Single center study | 75 | 35.0 | 86.0 | ACLF grade 2 and 3 were more frequent in MDRO infected patients |
| Cassini et al[3] | Meta-analysis | 671 689 | NA | 4.9 | Estimate the incidence of infections caused by selected antibiotic-resistant bactéria in countries of the EU and EEA in 2015 |
| Trebicka et al[9] | European multicenter | 376 | 18.9 | 40.8 at 28.0 d; 48.7 at 90.0 d | In infection-induced ACLF, the prevalence of MDR strains was significantly higher; severe sepsis (40.7% vs 21.6%), ACLF (72.3% vs 42.0%) and 90-d mortality (48.7% vs 30.7%) were more frequent in infections caused by MDR strains compared to non-MDR strains |
| Costabeber et al[17] | Retrospective | 474 | 37.5 | - | To evaluate the resistance profile of bacteria isolated from cirrhotic patients admitted to a referral hospital in Brazil |
| Trebicka et al[9] | European multicenter | 520 | 14.8 | 35.1 at 28.0 d | MDROs were not significantly different between specific infections in the different European regions; MDROs were more frequently isolated in the ICU (23.8% vs 12.2%) and nosocomial infections (21.3% vs 8.3% and 6.6% in CA and HCA infections, respectively); MDROs were more prevalent in infections causing severe sepsis/shock (30.3% vs 12.2%) or ACLF (20.5% vs 9.4%) |
| Johnson et al[18] | Retrospective | 3951 | 5.6 | 27.7 | Presence of MDR bacteria in the blood was not associated with in-hospital mortality |
In this review, we describe the epidemiology, clinical settings and the current evidence-based strategies for early recognition and treatment alternatives for MDR infection in cirrhotic patients.
Bacterial infections affect 25%-35% of hospitalized patients with cirrhosis and lead to a four-fold increase in their mortality when compared to noninfected counterparts[11]. The prognostic impact of infections is such that they are considered defining events of state 6 (end-state, late decompensation) in the clinical course of cirrhosis[12]. Moreover, infections (together with alcoholic hepatitis) are the most important drivers of acute decompensation of cirrhosis and acute-on-chronic liver failure (ACLF)[9,13,14].
Despite the evolution in medical care, there is evidence suggesting that mortality associated with infections in individuals with cirrhosis might be increasing. Our group, for instance, has demonstrated that mortality associated with spontaneous bacterial peritonitis increased from 22% to 40% in a decade[15,16]. An explanation for this finding might be the growing importance of infections caused by MDRO. In our setting, after evaluating 5800 isolates from hospitalized patients, we have shown that 38% and 44% of individuals with and without cirrhosis respectively were infected with MDRO. Furthermore, in that study, 20% of Escherichia coli and Klebsiella sp strains infecting patients with cirrhosis were extended-spectrum beta-lactamase (ESBL)-producing bacteria, and 44% of S. aureus strains were methicillin-resistant[17].
Additionally, a recent European study has clearly demonstrated that infections associated with MDRO are increasing in individuals with cirrhosis. The study evaluated two prospective multicenter cohorts of patients hospitalized for acute decompensation of cirrhosis or ACLF. The first cohort consisted of 1146 individuals evaluated in 2011, of which 39.7% were infected. The second cohort consisted of 883 individuals evaluated in 2018, of which 32.2% were infected. In that study, infections associated with MDRO were diagnosed in 29.2% of subjects with positive cultures in the 2011 cohort and in 37.9% of those pertaining to the 2018 cohort[10].
On a global level, another prospective cohort study has demonstrated the relevance of infections associated with MDRO in individuals with cirrhosis worldwide. The authors included 1302 infected patients with cirrhosis from 46 different centers in Europe, America and Asia. The most common infections were spontaneous bacterial peritonitis [spontaneous bacterial peritonitis (SBP), 27%], urinary tract infection (22%) and pneumonia (19%), and 57% of isolates consisted of Gram-negative bacteria. Among individuals with positive cultures, 34% were infected with MDRO, most commonly ESBL-producing Enterobacteriaceae, methicillin-resistant S. aureus, vancomycin-resistant Enterococci, Pseudomonas aeruginosa, and Acinetobacter baumannii. The prevalence of infections caused by MDRO was higher in Asia (51%), than in Europe (29%) or America (27%). Independent risk factors for infections with MDRO were being from Asia (and mostly from India, where MDRO were present in 73% of isolates), using antibiotics in the three months previous to hospital admission, being exposed to healthcare facilities, and the site of infection (pneumonia, skin and soft tissue infection and urinary tract infection had higher odds of being caused by MDRO)[7].
Africa and Oceania are poorly represented in studies evaluating the prevalence of MDRO in patients with cirrhosis. However, a recent retrospective cohort study has demonstrated a low prevalence of MDRO in blood cultures of patients with cirrhosis hospitalized in Australia (5.6% of admissions). Despite the low prevalence, the study has shown a significant increase in infections caused by MDRO over a decade[18], similarly to what had been previously verified in Europe[10].
Different abnormalities related to the immune system and the occurrence of bacterial translocation from the intestinal lumen increase the susceptibility to infections in cirrhotic patients[19]. Bacterial infections are very common in cirrhosis affecting approximately 1/3 of patients with decompensated cirrhosis and are responsible for significant mortality. In a review study Arvaniti et al[11] evaluated 178 studies with more than 11000 patients with cirrhosis and found that infections increase mortality four-fold. In these patients delayed antibiotic treatment and inadequate empirical therapy are independently associated with mortality[20,21].
As stated before, recent studies suggest that about 34% of cirrhotic patients with bacterial infections are infected with MDRO[7]. Infections by MDRO have a worse prognosis because they are associated with lower rate of infection resolution with traditional empirical antibiotic treatment. In the first series (2005-2007) of a Spanish study, failure to antibiotic treatment was higher (30% vs 8%) in MDR infections than in susceptible bacterial infections. In addition, this study found a higher frequency of septic shock (26% vs 10%) and higher hospital mortality rate (25% vs 12%) in MDR infections compared to infections by non-resistant bacteria. An important finding of the second series (2010-2011) of this study was the higher prevalence of MDRO in nosocomial infections (39%) compared to HCA (a type of infection that occurs in patients with a previous contact with a healthcare environment, e.g., hospitalization or short-term admission for at least 2 d in the previous 90 d, residence in a nursing home or a long-term care facility, or chronic hemodialysis) and community-acquired infections (20% and 0%, respectively)[22]. Similarly, an intercontinental study evaluated 1302 infected cirrhotic patients and confirmed that infections caused by MDRO were associated with a lower efficacy (40% vs 68%) and a longer duration (12 d[7-18] vs 10 d[7-15]) of empirical antibiotic treatment. Furthermore, patients with bacterial infections by multi-resistant strains had a higher incidence of septic shock (27% vs 13%) and higher in-hospital and 28-d mortality rate (31% vs 21% and 34% vs 22%, respectively)[7]. Finally, a meta-analysis on the impact of infections by MDRO on mortality in cirrhosis found a four times increased risk of mortality associated with bacterial resistance compared to non-resistant bacterial infections[11].
MDR infection results from an interaction of different risk factors that act synergistically. Although some risk factors have been identified, we still far from completely understand all the mechanisms involved, and there is still much to research in this field. Identifying risk factors for multidrug resistance is essential to define the best antibiotic treatment strategy in each case[21].
Previous use of antibiotics is a well-known driver for multidrug resistant infection in different clinical settings. A strong association of MDRO with previous antibiotic therapy was also observed in cirrhotic patients. Extended use of broad-spectrum antibiotics[23] and exposure to systemic antibiotics treatment for at least five days, especially in the previous three months, were highlighted as risk factors[7,24]. Prior use of beta-lactam antibiotics is especially important and has been identified as an independent predictor in the multivariate analysis[22,25]. These findings reinforce the importance of the judicious use of antibiotics in preventing MDRO emergence, with avoidance of overuse and early de-escalation strategies.
Another important risk factor for MDR infections is the previous occurrence of infections by resistant bacteria. In the multivariate analysis, infection caused by MDR bacteria in the last 6 mo increases the risk by 2.45 times[22].
Current or recent contact with the healthcare system is another important risk factor for MDR infections. A strong association between MDR infections and hospital admission has been shown in several studies[10,26,27]. Patients with nosocomial infection, hospitalization for more than 48 h, and those discharged in the last 30 d are at increased risk for MDR infections. In addition, an increased risk has been reported in patients admitted to the intensive care unit. The risk of MDR infections is also related to the duration of hospitalization and the invasiveness of the procedures performed.
Non-hospitalized patients with healthcare-associated (HCA) infections have an intermediate rate (14%-41%) of infections caused by MDRO, which is lower than in nosocomial infections (23%-39%) but higher than that observed in community-acquired infections (0%-16%). Therefore, the risk of MDR infection is directly related to where the infection was acquired (nosocomial or community-acquired)[22,26,28].
Although the association of quinolone prophylaxis with MDR infection was not identified with the use of norfloxacin for six months in a placebo-controlled trial[29] and in an epidemiological study[7], the prophylactic use of antibiotics has been pointed out as a risk factor for MDR infections in other studies[22,25,30].
The bacteriological profile of antibiotic resistance in each geographic region also influences the risk of multidrug resistance. Patients from India, other Asian centers, and South America had an increased risk of MDR bacteria[7]. The bacteriological profile of antibiotic resistance at each health care unit is also an important point, and this local antibiotic resistance profile should be considered in the estimation of the MDR risk of each patient[7,31].
MDR risk is also related to the site of the infection. For example, MDR infections were more commonly observed in patients with pneumonia, skin and soft tissue infections than in those with SBP or spontaneous bacteremia[7,27].
A higher prevalence of proton pump inhibitors (PPI) use among patients with MDR infection has been reported[24], suggesting that PPI could be a risk factor for infections caused by MDRO. However, this association still needs to be better explored in further studies.
MDR infections are more commonly observed in patients with worse liver function, however it is difficult to establish whether liver function is an independent risk factor for MDR because patients with more severe liver disease have more frequent hospitalizations and are more exposed to the use of antibiotics. In the study of Piano et al[7], liver function was not independently associated with MDR infections in the multivariate analysis although patients with MDR infections presented higher Child and MELD-Na scores. Therefore, even if it is not an independent risk factor, MDR infection is often associated with more severe liver disease.
Table 2 shows the main risk factors for MDR infections.
| No. | Risk factors for MDR infection in cirrhosis |
| 1 | Prior (3 mo) use of broad-spectrum antibiotics |
| 2 | Prior infection by MDROs (6 mo) |
| 3 | Nosocomial infection |
| 4 | Recent contact with the healthcare system |
| 5 | Site of infection (pneumonia, skin, and soft tissue infections) |
| 6 | Geographic region |
| 7 | Prophylactic use of antibiotics (?)/proton pump inhibitors use? |
In the early phases of bacterial infections in patients with cirrhosis, typical signs of infection (like fever) may not be present. Bacterial infections can precipitate and/or constitute part of the process of acute decompensation of cirrhosis, and an appropriate work-up for infections (e.g., diagnostic paracentesis; chest X-ray; urinalysis; blood, ascites and urine cultures) should be made in all patients hospitalized for decompensated liver disease[32].
Optimizing the prescription of antibiotics is cardinal to effectively treat infections, protect patients from harms caused by unnecessary antibiotic use, and combat antibiotic resistance. As the prevalence of MDRO differs throughout the world, the choice of an empirical antibiotic therapy should be tailored to the local microbiological epidemiology, and it should also be influenced by the type of infection (e.g., SBP, urinary tract infection, pneumonia, soft tissue infection), the severity of infection, and the potential risk factors for infections caused by MDRO[32,33] (Table 1).
Effective antibiotics need to be administered as early as possible. In a retrospective cohort study of 126 cirrhotic patients with SBP-associated septic shock, each hour of delay in the appropriate antimicrobial therapy was associated with 1.86 times increase in hospital mortality[34]. In a worldwide study of hospitalized patients with cirrhosis, the administration of adequate empirical antibiotic treatment was found to be an independent and the only potentially modifiable predictor of in-hospital and 28-d mortality[7].
In the whole Canonic series, MDROs were more prevalent in infections causing severe sepsis/septic shock and/or ACLF and associated to lower resolution rate and higher mortality at 28 d, especially if treated with inadequate empirical antibiotic strategies[10]. A multicenter retrospective study of 865 consecutive patients with a first presentation of SBP in Korea pointed that empirical carbapenem treatment was significantly associated to lower in-hospital mortality than third-generation cephalosporins of among 314 critically ill patients (CLIF-SOFA scores ≥ 7; 23.1% vs 38.8%; aOR, 0.84; 95%CI, 0.75-0.94; P = 0.002)[35].
In the treatment of HCA infections in an Italian population of cirrhotic patients, with a prevalence of MDRO of 40%-46%, empirical broad-spectrum therapy (imipenem/cilastatin ± vancomycin) significantly reduced in-hospital mortality when compared to third generation cephalosporins, particularly in patients with sepsis. It also reduced the rate of treatment failure and length of stay[36]. In a prospective randomized controlled trial (RCT) that enrolled 32 patients with nosocomial SBP, the broad-spectrum antibiotic therapy (meropenem plus daptomycin) was more effective than ceftazidime (86.7% vs 25%, P < 0.001). Furthermore, meropenem plus daptomycin was effective in 90% of nonresponders to ceftazidime. The response to first-line treatment was an independent predictor of survival[37].
The isolation of MDRO in rectal and nasal swabs could also guide empirical antibiotic strategies in cirrhotic patients. In a study of two European cohorts comprising a total of 907 critically ill patients, including 550 patients with cirrhosis, rectal colonization by MDRO was highly prevalent in cirrhotic patients, ranging from 28.7% to 31.1% in intensive care unit admissions, and MDRO carriage increased the short-term risk of subsequent infection by the colonizing organism[38].
Patients with cirrhosis and severe infections may benefit from therapeutic strategies aimed at optimizing the antibiotics’ pharmacokinetic/pharmacodynamic target. The use of high antibiotic doses within the first 48-72 h after the diagnosis of infection and the continuous or extended infusions of beta-lactams are more likely to achieve and to maintain serum drug levels above the minimum inhibitory concentration compared to standard bolus administration 6. In a secondary analysis of a European prospective multicenter study of patients with cirrhosis and bloodstream infection, the empirical continuous/extended infusion of piperacillin-tazobactam or carbapenems was associated with lower mortality compared to traditional dosing schedules (adjusted hazard ratio, 0.41; 95%CI, 0.110-0.936; P = 0.04), and it was particularly useful in those patients who were critically ill[39].
After completing 48-72 h of antibiotic therapy, early de-escalation can be considered based on clinical evolution and available antibiotic susceptibility tests. Short-term treatment is another key measure to prevent antibiotic resistance[7,40,41]. In the 1990s, a randomized controlled trial of 100 patients with SBP showed that a short-course (5-d) treatment is as effective as long-course (10-d) therapy and significantly less expensive[42]. In non-SBP infections, the optimal duration of antibiotic therapy has not been established, but data from the general population suggests that a 7-d course is adequate for most infections[32].
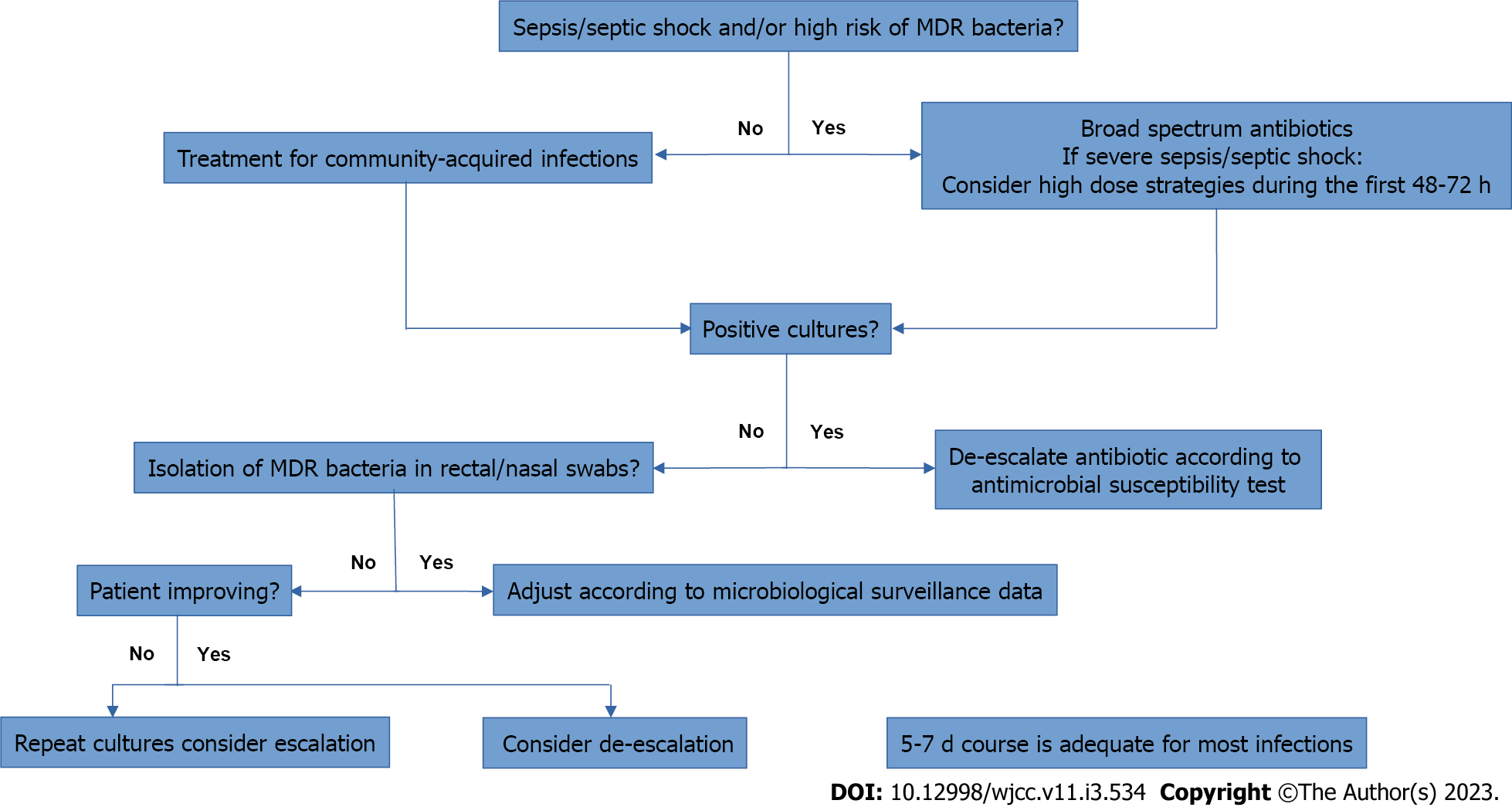
Figure 1 and Table 3 summarizes the management of patients with cirrhosis and bacterial infection.
| Type of infection | Community-acquired infection | Nosocomial and HCA infection or sepsis |
| SBP, spontaneous bacterial empyema and spontaneous bacteremia | Cefotaxime or Amoxicilin/clavulanic acid | Piperacillin/tazobactam or Meropenem ± Vancomycin or Daptomycin or Linezolid1 |
| UTI | Fosfomycin or cotrimoxazole | Uncomplicated: Nitrofurantoin or Fosfomycin; if sepsis: Piperacillin/tazobactam or Meropenem ± Glycopeptide |
| Pneumonia | Amoxicilin/clavulanic acid; Ceftriaxone + Macrolide; Levofloxacin; Moxifloxacin | Piperacillin/tazobactam or Meropenem or Ceftazidime + Ciprofloxacin; Glycopeptides or Linezolid1 should be added in patients with risk factors for MRSA2 |
| Skin and soft tissue infections | Amoxicilin/clavulanic acidor ± Clindamycin | Meropenem or Piperacillin/tazobactam + Glycopeptide or Daptomycin or Linezolid1 ± Cindamycin; if necrotizing fascitis: Meropenem + Daptomycin + Clindamycin |
The emergence and spread of MDRO in cirrhosis require the implementation of measures aimed to prevent its complications. Pharmacological and non-pharmacological strategies are needed, including hand hygiene and barrier precaution, restriction of antibiotic use to high-risk populations, de-escalating antibiotic therapy based on rapid microbiological tests, study of non-antibiotic prophylaxis measures, broad and strict infection control policies, and programs of epidemiological surveillance[25].
Non-antibiotic drugs with potential benefit on infections in cirrhosis: Although antibiotic treatment is the most effective measure for controlling established MDR infection, other drugs have shown potential benefits in preventing infections in cirrhosis. A lower occurrence of SBP has been demonstrated in patients using non-selective beta-blockers (NSBB)[43]. This benefit of NSBB has been related to its potential effect in improving intestinal motility, improving intestinal permeability, and reducing bacterial translocation[44,45]. Statins also seem to have a beneficial effect against bacterial infection[46] that is attributed to its anti-inflammatory and immunomodulatory properties.
Non-pharmacological measures: Non-pharmacological measures are based on preventive strategies and procedures focusing on intestinal colonization with MDRO. The most important preventive measure is the restrictive and judicious antibiotic use since the main driver for the emergence of MDR infections is the widespread use of antibiotics.
The main non-pharmacological measures focus on gut microbiota[27]. A healthy microbiome is essential to prevent colonization and infection by MDRO[25]. Different approaches focusing on the modulation of the intestinal microbiome were studied, such as probiotics, prebiotics/synbiotics dietary regimens, and fecal microbiota transplant (FMT)[45].
Although some studies have shown favorable results with probiotics, there are also negative studies. This controversy is probably related to different probiotics used and the different number and concentration of the species. Further studies are necessary to define the ideal combination, dose, and duration of administration.
FMT involves the safe transfer of exogenous bacterial flora from a healthy donor to another patient, in capsule or liquid formulations. The rationale for using this technique is the central role of the gastrointestinal colonization in the development of MDR infections. It has been demonstrated that colonization by MDRO is associated with increased risk of infection by the colonizing bacteria in the short-term[38]. FMT has the potential effect of promoting MDRO decolonization.
A systematic review with meta-analysis[47] of five studies, with a total number of 52 patients, evaluated whether FMT decolonizes antibiotic-resistant bacteria from the gut of colonized adults. Evidence from this meta-analysis indicates a potential benefit of FMT as a decolonization intervention, with few adverse effects. Despite the low quality of evidence appointed by this meta-analysis, these preliminary results suggest that FMT is a promising approach that deserves further analysis in RCTs.
In cirrhotic patients, a preliminary study of FMT in patients with advanced cirrhosis on lactulose and rifaximin demonstrated that FMT restored antibiotic-associated disruption in microbial diversity and function[48]. The impact of FMT in the reduction of gut microbial antibiotic resistance genes was later reported in two trials: a capsule FMT trial and an enema FMT trial with 20 patients each. This study demonstrated that, despite differences in routes of administration, antibiotic resistance gene abundance was reduced after FMT compared to pre-FMT baseline and non-FMT groups in decompensated cirrhosis[49].
Another possible approach, although still requiring further studies, is phage therapy. This technique is based on the use of bacteriophages which are viruses that infect bacteria[50]. The bacteriophages replicate inside the bacteria leading to their destruction. It is an old technique that was left aside with the advent of antibiotics, but nowadays it has been considered again as a therapeutic option to face the serious problem of antibiotic multidrug resistance[51,52]. Although specific studies in cirrhotic patients are not available, phage therapy represents a possible future alternative therapy for controlling MDRO[53].
In this review, we describe the epidemiology, clinical settings and the current evidence-based strategies for early recognition and treatment alternatives for MDR infection in cirrhotic patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bukhari SM, Pakistan; Kreisel W, Germany; Sahle Z, Ethiopia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6072] [Cited by in F6Publishing: 7479] [Article Influence: 575.3] [Reference Citation Analysis (0)] |
| 2. | Kadri SS. Key Takeaways From the U.S. CDC's 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit Care Med. 2020;48:939-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 3. | Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL; Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1615] [Cited by in F6Publishing: 1622] [Article Influence: 324.4] [Reference Citation Analysis (0)] |
| 4. | Weist K, Högberg LD. ECDC publishes 2015 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Euro Surveill. 2016;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Serra-Burriel M, Keys M, Campillo-Artero C, Agodi A, Barchitta M, Gikas A, Palos C, López-Casasnovas G. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS One. 2020;15:e0227139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 6. | Fernández J, Piano S, Bartoletti M, Wey EQ. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J Hepatol. 2021;75 Suppl 1:S101-S117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 8. | Fischer P, Pandrea S, Dan Grigorescu M, Stefanescu H, Tefas C, Hadade A, Procopet B, Ionescu D. The threat of carbapenem resistance in Eastern Europe in patients with decompensated cirrhosis admitted to intensive care unit. Dig Liver Dis. 2022;54:1385-1391. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 9. | Trebicka J, Fernandez J, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. Reply to: Correspondence on 'The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology'. J Hepatol. 2021;74:480-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 10. | Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V; European Foundation for the Study of Chronic Liver Failure (EF-Clif). Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 11. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 720] [Cited by in F6Publishing: 749] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 12. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 281] [Article Influence: 46.8] [Reference Citation Analysis (1)] |
| 13. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1720] [Cited by in F6Publishing: 1866] [Article Influence: 169.6] [Reference Citation Analysis (3)] |
| 14. | O'Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, Subramanian RM, Kamath PS, Thuluvath P, Vargas HE, Maliakkal B, Tandon P, Lai J, Thacker LR, Bajaj JS. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 2018;67:2367-2374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 169] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 15. | Coral G, de Mattos AA, Damo DF, Viégas AC. [Prevalence and prognosis of spontaneous bacterial peritonitis. Experience in patients from a general hospital in Porto Alegre, RS, Brazil (1991-2000)]. Arq Gastroenterol. 2002;39:158-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Musskopf MI, Fonseca FP, Gass J, de Mattos AZ, John JA, de Mello Brandão AB. Prognostic factors associated with in-hospital mortality in patients with spontaneous bacterial peritonitis. Ann Hepatol. 2012;11:915-920. [PubMed] [Cited in This Article: ] |
| 17. | Costabeber AM, Mattos AA, Sukiennik TC. Prevalence of bacterial resistance in hospitalized Cirrhotic patients in southern brazil: A new challenge. Rev Inst Med Trop Sao Paulo. 2016;58:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Johnson AL, Ratnasekera IU, Irvine KM, Henderson A, Powell EE, Valery PC. Bacteraemia, sepsis and antibiotic resistance in Australian patients with cirrhosis: a population-based study. BMJ Open Gastroenterol. 2021;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Thulstrup AM, Sørensen HT, Schønheyder HC, Møller JK, Tage-Jensen U. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis. 2000;31:1357-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 20. | Bartoletti M, Giannella M, Lewis R, Caraceni P, Tedeschi S, Paul M, Schramm C, Bruns T, Merli M, Cobos-Trigueros N, Seminari E, Retamar P, Muñoz P, Tumbarello M, Burra P, Torrani Cerenzia M, Barsic B, Calbo E, Maraolo AE, Petrosillo N, Galan-Ladero MA, D'Offizi G, Bar Sinai N, Rodríguez-Baño J, Verucchi G, Bernardi M, Viale P; ESGBIS/BICHROME Study Group. A prospective multicentre study of the epidemiology and outcomes of bloodstream infection in cirrhotic patients. Clin Microbiol Infect. 2018;24:546.e1-546.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D, Wood G, Dial S, Zanotti S, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56:2305-2315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, Seva-Pereira T, Corradi F, Mensa J, Ginès P, Arroyo V. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 23. | Zhong L, Men TY, Li H, Peng ZH, Gu Y, Ding X, Xing TH, Fan JW. Multidrug-resistant gram-negative bacterial infections after liver transplantation - spectrum and risk factors. J Infect. 2012;64:299-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Figueiredo LM, Rafael MA, Alexandrino G, Branco JC, Carvalho R, Costa MN, Martins A. Risk factors for the emergence of multidrug-resistant organisms in liver cirrhosis. Gastroenterol Hepatol. 2022;45:186-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Fernández J, Bert F, Nicolas-Chanoine MH. The challenges of multi-drug-resistance in hepatology. J Hepatol. 2016;65:1043-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 580] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 27. | Gallaher CE, Shawcross DL. Management of Multidrug-Resistant Infections in Cirrhosis. Semin Liver Dis. 2022;42:173-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Moreau R, Elkrief L, Bureau C, Perarnau JM, Thévenot T, Saliba F, Louvet A, Nahon P, Lannes A, Anty R, Hillaire S, Pasquet B, Ozenne V, Rudler M, Ollivier-Hourmand I, Robic MA, d'Alteroche L, Di Martino V, Ripault MP, Pauwels A, Grangé JD, Carbonell N, Bronowicki JP, Payancé A, Rautou PE, Valla D, Gault N, Lebrec D; NORFLOCIR Trial Investigators. Effects of Long-term Norfloxacin Therapy in Patients With Advanced Cirrhosis. Gastroenterology. 2018;155:1816-1827.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Salerno F, Borzio M, Pedicino C, Simonetti R, Rossini A, Boccia S, Cacciola I, Burroughs AK, Manini MA, La Mura V, Angeli P, Bernardi M, Dalla Gasperina D, Dionigi E, Dibenedetto C, Arghittu M; AISF Investigators. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017;37:71-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Aguirre-García J. Comment on: Ruelas-Villavicencio A L, et. al. "In whom, how and how often is surveillance for hepatocellular carcinoma cost-effective? Ann Hepatol. 2005;4:289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Piano S, Tonon M, Angeli P. Changes in the epidemiology and management of bacterial infections in cirrhosis. Clin Mol Hepatol. 2021;27:437-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Miranda-Zazueta G, León-Garduño LAP, Aguirre-Valadez J, Torre-Delgadillo A. Bacterial infections in cirrhosis: Current treatment. Ann Hepatol. 2020;19:238-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Karvellas CJ, Abraldes JG, Arabi YM, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Kim SW, Yoon JS, Park J, Jung YJ, Lee JS, Song J, Lee HA, Seo YS, Lee M, Park JM, Choi DH, Kim MY, Kang SH, Yang JM, Song DS, Chung SW, Kim MA, Jang HJ, Oh H, Lee CH, Lee YB, Cho EJ, Yu SJ, Kim YJ, Yoon JH, Lee JH. Empirical Treatment With Carbapenem vs Third-generation Cephalosporin for Treatment of Spontaneous Bacterial Peritonitis. Clin Gastroenterol Hepatol. 2021;19:976-986.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Merli M, Lucidi C, Di Gregorio V, Lattanzi B, Giannelli V, Giusto M, Farcomeni A, Ceccarelli G, Falcone M, Riggio O, Venditti M. An empirical broad spectrum antibiotic therapy in health-care-associated infections improves survival in patients with cirrhosis: A randomized trial. Hepatology. 2016;63:1632-1639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, Cavallin M, Gola E, Sticca A, Loregian A, Palù G, Zanus G, Senzolo M, Burra P, Cillo U, Angeli P. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology. 2016;63:1299-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 38. | Prado V, Hernández-Tejero M, Mücke MM, Marco F, Gu W, Amoros A, Toapanta D, Reverter E, Peña-Ramirez C, Altenpeter L, Bassegoda O, Mezzano G, Aziz F, Juanola A, Rodríguez-Tajes S, Chamorro V, López D, Reyes M, Hogardt M, Kempf VAJ, Ferstl PG, Zeuzem S, Martínez JA, Vila J, Arroyo V, Trebicka J, Fernandez J. Rectal colonization by resistant bacteria increases the risk of infection by the colonizing strain in critically ill patients with cirrhosis. J Hepatol. 2022;76:1079-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Bartoletti M, Giannella M, Lewis RE, Caraceni P, Tedeschi S, Paul M, Schramm C, Bruns T, Merli M, Cobos-Trigueros N, Seminari E, Retamar P, Muñoz P, Tumbarello M, Burra P, Torrani Cerenzia M, Barsic B, Calbo E, Maraolo AE, Petrosillo N, Galan-Ladero MA, D'Offizi G, Zak-Doron Y, Rodriguez-Baño J, Baldassarre M, Verucchi G, Domenicali M, Bernardi M, Viale P; ESGBIS/BICHROME study group. Extended Infusion of β-Lactams for Bloodstream Infection in Patients With Liver Cirrhosis: An Observational Multicenter Study. Clin Infect Dis. 2019;69:1731-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Fernández J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology. 2016;63:2019-2031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 41. | Allaire M, Cadranel JF, Nguyen TTN, Garioud A, Zougmore H, Heng R, Perignon C, Ollivier-Hourmand I, Dao T. Management of infections in patients with cirrhosis in the context of increasing therapeutic resistance: A systematic review. Clin Res Hepatol Gastroenterol. 2020;44:264-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology. 1991;100:1737-1742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 196] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, Burroughs AK. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int. 2009;29:1189-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 44. | Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H; Vienna Hepatic Hemodynamic Lab. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 45. | Yan K, Garcia-Tsao G. Novel prevention strategies for bacterial infections in cirrhosis. Expert Opin Pharmacother. 2016;17:689-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Motzkus-Feagans C, Pakyz AL, Ratliff SM, Bajaj JS, Lapane KL. Statin use and infections in Veterans with cirrhosis. Aliment Pharmacol Ther. 2013;38:611-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Tavoukjian V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: a systematic review and meta-analysis. J Hosp Infect. 2019;102:174-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Bajaj JS, Kakiyama G, Savidge T, Takei H, Kassam ZA, Fagan A, Gavis EA, Pandak WM, Nittono H, Hylemon PB, Boonma P, Haag A, Heuman DM, Fuchs M, John B, Sikaroodi M, Gillevet PM. Antibiotic-Associated Disruption of Microbiota Composition and Function in Cirrhosis Is Restored by Fecal Transplant. Hepatology. 2018;68:1549-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 49. | Bajaj JS, Shamsaddini A, Fagan A, Sterling RK, Gavis E, Khoruts A, Fuchs M, Lee H, Sikaroodi M, Gillevet PM. Fecal Microbiota Transplant in Cirrhosis Reduces Gut Microbial Antibiotic Resistance Genes: Analysis of Two Trials. Hepatol Commun. 2021;5:258-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 50. | Principi N, Silvestri E, Esposito S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front Pharmacol. 2019;10:513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 51. | Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014;8:129-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 52. | Kortright KE, Chan BK, Koff JL, Turner PE. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe. 2019;25:219-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 53. | Fauconnier A, Nagel TE, Fauconnier C, Verbeken G, De Vos D, Merabishvili M, Pirnay JP. The Unique Role That WHO Could Play in Implementing Phage Therapy to Combat the Global Antibiotic Resistance Crisis. Front Microbiol. 2020;11:1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |