Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11827
Peer-review started: June 30, 2022
First decision: August 4, 2022
Revised: August 11, 2022
Accepted: September 19, 2022
Article in press: September 19, 2022
Published online: November 16, 2022
Paraneoplastic neurological syndrome (PNS) is an unusual event. PNS caused by cystitis glandularis (CG) or a bladder tumor is extremely rare; hence, missed diagnosis or misdiagnosis can easily occur. To date, approximately 21 cases have been reported in PubMed.
We report a case of PNS caused by CG and describe the clinical and imaging features. The main clinical feature was advanced cognitive impairment, and early clinical features were memory impairment, decreased computational ability, and abnormal behavior. Later clinical features were dementia, vomiting, inability to eat and walk, urinary incontinence, and hematuria. Imaging features on cranial magnetic resonance imaging were diffuse white matter lesions. Paraneoplastic tumor markers were normal. A total abdominal computed tomography scan showed multiple thickened areas on the bladder wall with local prominence. Cystoscopy revealed a volcanic protuberance on the posterior wall of the bladder with a diameter of 6 cm and no pedicle. The postoperative pathological diagnosis was CG. The patient recovered well following resection of CG. PNS cases caused by previous bladder tumors can be retrieved from PubMed to describe the clinical signs and prognosis of PNS.
The main clinical feature of PNS caused by CG was dementia, and the imaging features were diffuse cerebral white matter lesions. Resection of CG lesions is the fundamental treatment for PNS induced by CG. This case highlights the impor
Core Tip: Paraneoplastic neurological syndrome (PNS) caused by a bladder tumor is an unusual event, and PNS caused by cystitis glandularis (CG) is extremely rare.CG is considered a precancerous lesion of bladder cancer. In this case combined with an analysis of cases indexed in PubMed, the main clinical feature of PNS caused by CG is advanced cognitive impairment. The imaging features on cranial magnetic resonance imaging are diffuse white matter lesions. Resection of CG lesions is the fundamental treatment for PNS induced by CG. However, most clinicians did not consider that PNS was caused by CG and gave symptomatic supportive treatment, which resulted in long delays in diagnosis and appropriate treatment.
- Citation: Zhao DH, Li QJ. Paraneoplastic neurological syndrome caused by cystitis glandularis: A case report and literature review. World J Clin Cases 2022; 10(32): 11827-11834
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11827.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11827
Paraneoplastic neurological syndrome (PNS) comprises a group of clinical syndromes that occur in the presence of systemic malignant tumors[1-3]. PNS can affect any part of the central and peripheral nervous system[4-6]. Most paraneoplastic syndromes are caused by autoimmune cross-reactions between tumor antigens and proteins expressed in the nervous system[7-11]. PNS is rare, affecting less than 0.6-1/10000 patients with cancer[12,13]. The Lambert-Eaton myasthenic syndrome which occurring in about 1% of patients with small cell lung cancer is relatively frequent[14]. Various antibodies associated with PNS have been reported, defining different subtypes of PNS; however, approximately one-third of patients do not have detectable antibodies and 5%-10% have an atypical antibody that is not well-characterized[15]. Even in patients with highly suspected PNS, only 6.9% of patients were positive for tumor neuroantibodies[16].
PNS may antedate the diagnosis of primary tumors in approximately 60%-80% of PNS patients, and antedate the diagnosis of cancer by several months to several years[17]. Symptoms of PNS can develop rapidly or slowly[15]. After diagnosing PNS, the risk of cancer development significantly decreases in two years and is very low after four years[17].
Early clinical manifestations of PNS are atypical, and most are non-specific symptoms[18]. PNS associated with a bladder tumor has rarely been reported, and could represent paraneoplastic encephalopathy, paraneoplastic cerebellar degeneration, paraneoplastic opsoclonus-ataxia syndrome, neuromyelitis optica, stiff person syndrome, and other symptoms, such as visual changes, glossal spasm and dysphagia[19-24]. However, paraneoplastic syndromes associated with bladder tumors rarely lead to cognitive impairment, and are easily misdiagnosed as other diseases. Therefore, there is an urgent need to better understand the characteristics of PNS caused by cystitis glandularis (CG), which is a benign non-neoplastic disease with a potential malignant tendency of bladder mucosal epithelial hyperplasia. In the present case, the main clinical feature was dementia, and tumor neuroantibodies were negative. The patient was misdiagnosed with cerebrovascular disease in 2016, and was subsequently found to have CG. The patient recovered well following resection of CG.
The patient, a 69-year-old Chinese woman, was admitted to hospital with the complaints of memory loss for one year and nausea and vomiting for four days.
One year previously, the patient began to suffer from memory disorder, and her main clinical manifestation was forgetting recent events. She could not find her way home after going out alone, experienced a loss of calculating power, and used the wrong money for shopping. She gradually showed abnormal behavior, such as repeating requests to return to her original house to find something and simultaneously wearing dirty and clean clothes after bathing. She was diagnosed with cerebral small vessel disease and vascular dementia in another hospital. Her treatment consisted of regulating her blood lipids, loweringher blood pressure, and providing antiplatelet therapy, but her symptoms did not improve. One month prior to admission, she developed intermittent involuntary twitching of the right index finger for a few seconds at a time. Four days before admission, the patient developed nausea and non-projectile vomiting, and her vomitus was biliary fluid. Two days previously, she visited the local hospital, where she underwent head and chest computed tomography (CT) scans that showed bilateral basal ganglia infarction, demyelination of bilateral paraventricular white matter, brain atrophy, slight inflammation and multiple small nodules in both lungs, and bilateral pleural thickening. The symptoms of nausea and vomiting did not improve following treatment with acid suppression and stomach protection. Due to aggravation of symptoms and ineffective treatment she was hospitalized.
The patient was a primary school teacher, otherwise healthy, a nonsmoker and without any medical history of disease.
The patient denied a family history of malignant tumors.
Physical examination revealed a body temperature of 36.4°C, pulse of 92 bpm, a respiratory rate of 18 breaths/min, and blood pressure of 152/96 mmHg. She was conscious, but indifferent. Her pupils were equally large (2.5 mm in diameter) and sensitive to light. When she stared to the left, a small horizontal nystagmus could be seen. Her bilateral frontal lines and nasolabial grooves were symmetrical, and her tongue was in the middle. Her limbs had normal muscle strength and tension. Her bilateral deep and shallow sensations were symmetrical, and her bilateral Babinski sign was negative. Her rallying movement was steady. Her neck was soft, and her meningeal irritation sign was negative. An advanced cognitive function test showed that her orientation of person, time, and place was normal, but her memory, calculation, and judgment abilities were decreased. Her Mini-Mental State Examination (MMSE) score was 21 and included a 3-point reduction in recall, a 5-point reduction in computational power, and a 1-point reduction in judgment.
After admission, a routine urine test showed urinary protein 3+, urinary nitrite +, urinary occult blood 3+, and 92 red blood cells/μL under microscope. Her blood electrolytes showed a potassium level of 2.60 mmol/Land chlorine level of 95.0 mmol/L. Her blood potassium level fluctuated between 2.60 and 3.70 mmol/L; however, this level was normal after vomiting stopped. Her total cholesterol level was 7.04 mmol/L, and her low-density lipoprotein level was 5.17 mmol/L. Her myocardial zymogram showed a lactate dehydrogenase level of 633.0 U/L, homocysteine level of 32.7 μmol/L, and folic acid level of 2.67 μg/L. Her fasting blood sugar level, glycated hemoglobin level, renal function, HIV status, syphilis status, and hepatitis C status were normal. Paraneoplastic tumor markers (anti-Hu antibody, anti-Ri antibody, anti-Yo antibody, anti-amphiphysin antibody, anti-Tr antibody, anti-BAD antibody, anti-CV2 antibody, anti-ANN-3 antibody, anti-PCA-2 antibody, anti-Ma antibody, anti-Ma2/Ta antibody, anti-glutamate receptor NMDA/A1/A2 antibody, anti-GABA B receptor antibody, anti-LGI-1 antibody, andanti-CASPR2 antibody) were normal. Her thyroid function and antibodies, parathyroid hormone, and vitamin B12 levels were normal. Color Doppler ultrasound of her neck blood vessels showed atherosclerotic plaque formation in the left common carotid artery. Color Doppler ultrasound of her heart and electrocardiogram were normal. She underwent lumbar puncture, her cerebrospinal fluid pressure was 130 mmH2O, and routine cerebrospinal fluid, biochemical, and immunoglobulin tests were normal. An EEG showed mild-to-moderate abnormalities, and no spikes or-slow complex waves were found.
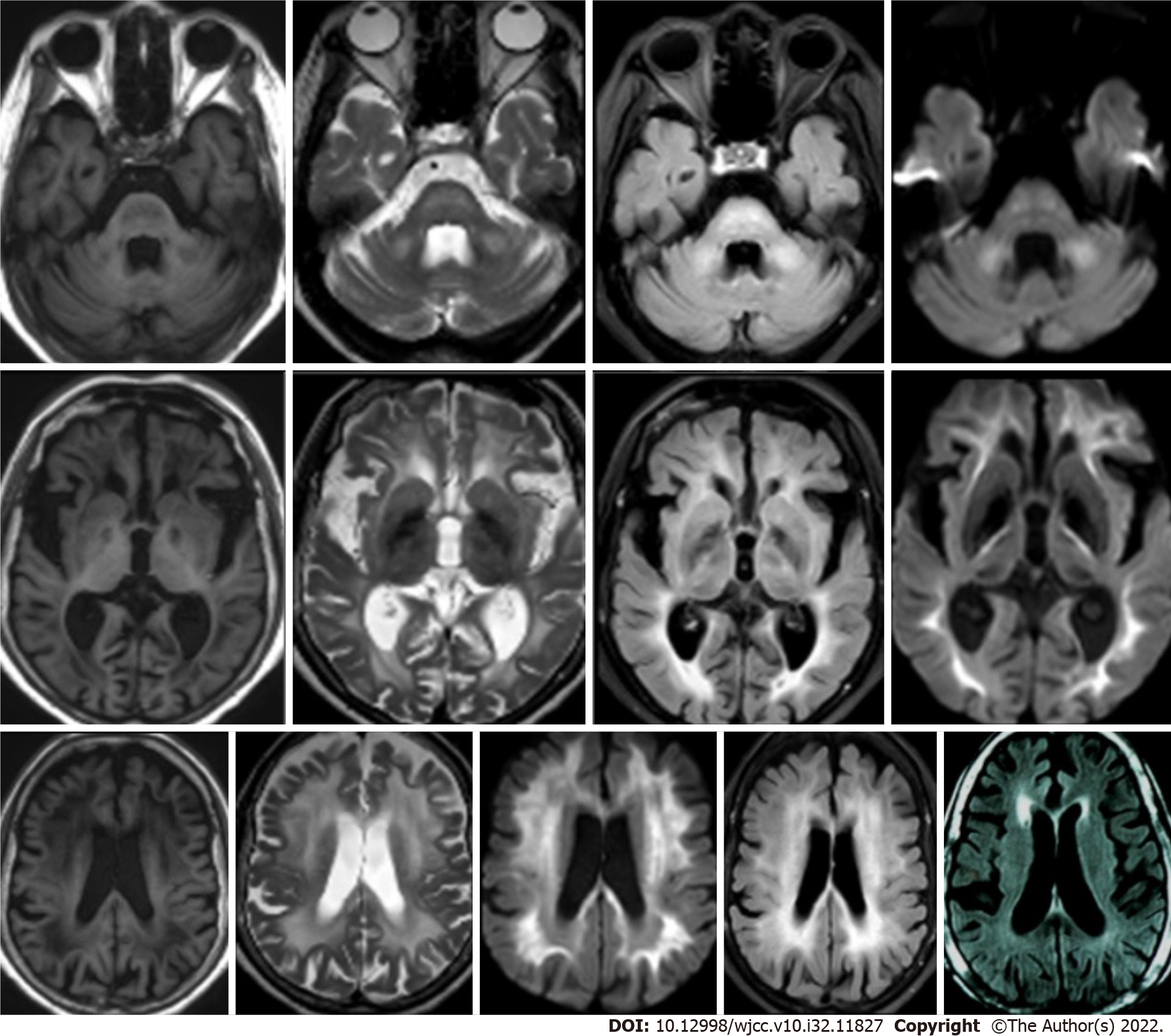
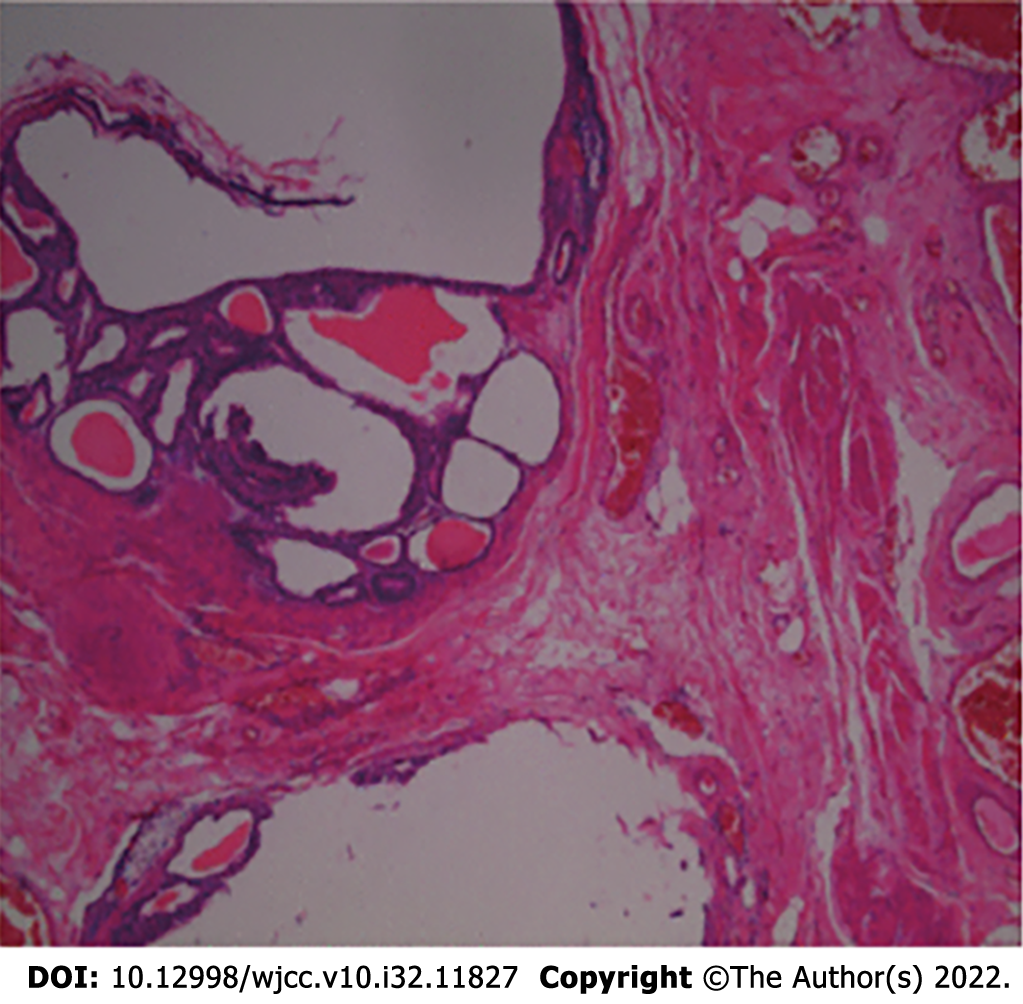
A gastroscopy suggested moderate chronic gastritis and antral mucosal erosion. Cranial magnetic resonance imaging (MRI) showed that her bilateral cerebral hemisphere white matter, brain stem and pons arms were symmetrically flaky, they also had a long T1and T2 sequence signal, a high fluid-attenuated inversion recovery sequence signal, and a high diffusion weighted image sequence signal (Figure 1). A total abdominal CT scan showed multiple thickened areas on the bladder wall with local prominence; multiple cysts in the liver, multiple cysts in the right kidney, and a calcified focus in the spleen. Cystoscopy revealed a volcanic protuberance on the posterior wall of the bladder with a diameter of 6 cm and no pedicle. The pathological diagnosis was CG (Figure 2).
As small cerebrovascular disease was first considered, treatment consisted of blood pressure control, donepezil hydrochloride, and statin treatment for lipid lowering and plaque stabilization was initiated. However, this treatment had no beneficial effect. The patient was also treated continuously with vitamin B1, vitamin B2, vitamin B6, and methyl cobalamin; however, her symptoms did not change, which ruled out Wernicke's encephalopathy. She received an infusion of methylprednisolone 500 mg for five days, which was gradually discontinued. The patient's symptoms, including giggling, ignorance of family, persistent vomiting, inability to eat and walk, and incontinence deteriorated. Her cognitive function worsened, and she could not cooperate to complete the MMSE.
As the patient had CG on the posterior wall of the bladder, which was considered a precancerous lesion, a partial cystectomy was performed on the 31st day after admission. On the second week after surgery, the symptoms of nausea and vomiting were alleviated and the indwelling catheter was removed. The patient was able to eat, and her finger shaking disappeared. On the sixth week after surgery, the patient was able to get out of bed, walk with a stick, and went to the toilet by herself. Her memory also recovered significantly. Her MMSE score was 26, her recall decreased by 3 points, and her computational ability decreased by 1 point.
Combined with the patient's history, pathology and good recovery after surgery, the final diagnosis was PNS caused by CG.
Postoperatively, the patient recovered well and was discharged 6 wk after operation. The patient did not require any medication.
During follow-up at 3 mo, 1 year, and 4 years, the patient was found to have recovered to her pre-morbidity state and was able to care for herself in daily life. No malignant tumors were found. Two years after treatment, a review of cranial MRIs in other hospitals revealed white matter lesions and brain atrophy (Figure 1).
PNS is a group of syndromes caused by malignant tumors with distant nervous system disorders. The neurological symptoms of PNS may occur 1-3 years before the onset of tumors. Common PNS symptoms include paraneoplastic encephalomyelitis, paraneoplastic marginal lobe encephalitis, paraneoplastic cerebellar degeneration, myoclonus, paraneoplastic strabismus ocular clonus, paraneoplastic retinal degeneration, subacute sensory neuropathy, paraneoplastic peripheral neuropathy, paraneoplastic stiff person syndrome, and myasthenia syndrome. Typical paraneoplastic antibodies are neuron antigen spectrum antibodies that are significant in the diagnosis of PNS[7,25].
The morbidity of CG ranges from 0.1% to 1.9%. Women are more frequently affected than men, and tends to occur in middle-aged and elderly people.CG lacks typical clinical manifestations, and mainly includes frequent micturition, urgent micturition, painful micturition, enuresis, urinary incontinence, increased nocturia, dysuria, macroscopic or microscopic hematuria and other symptoms. The pathology of CG is mainly hyperproliferation of basal cells of mucosal epithelium, which results in the lamina propria forming a solid epithelial cell nest. Glandular metaplasia occurs in the cell nest, forming glandular cystitis.
CG is considered to be a precancerous lesion with potential malignancy of glandular bladder cancer[26,27]. CG shows high expression of p53, Ki67, nmp-9 and other tumor markers[27]. Pantuck analyzed various samples of abnormal metaplasia of bladder epithelial tissues and various types of bladder cancer. He found that CG could be a precancerous lesion of bladder transitional cell carcinoma, and the period from CG to canceration was about 3 years[28]. The PNS of transitional bladder cell carcinoma, including that of paraneoplastic encephalomyelitis, subacute sensory neuropathy, cerebellar degeneration, strabismus, polymyositis, autonomic neuropathy, and paraneoplastic dermatomyositis, is mostly associated with anti-Hu and anti-Ri antibodies[24,29,30]. Even when paraneoplastic antibodies are negative, PNS secondary to bladder transitional cell carcinoma can be confirmed by the relief of PNS symptoms after resection of the tumors[31]. Although many types of paraneoplastic antibodies have been described, less than 50% of PNS patients have detected paraneoplastic antibodies[13,32-34]. Thus, the absence of paraneoplastic antibodies cannot rule out the diagnosis of PNS[15]. Following CG resection, our patient's cognitive dysfunction gradually recovered, and she was completely self-sufficient. Her symptoms did not recur within 4 years of follow-up. Although the patient's paraneoplastic antibodies were negative, it is suggested that CG was still the cause of her PNS.
Although PNS caused by GC is a very rare entity, combining our case with the cases reported in PubMed, we describe the clinical features of PNS caused by GC, which include cognitive impairment, abnormal behavior, vomiting, urinary incontinence, hematuria, negative tumor markers and paraneoplastic markers. Symptoms of cognitive impairment were alleviated after CG resection. This case highlights the importance of etiological treatment, as symptomatic treatment was ineffective. However, it was not clear how PNS was induced by CG or which type of antigen or antibody induced across-immune reaction with the nervous system.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masaru T, Hungary; Papazafiropoulou A, Greece S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 531] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 2. | Grativvol RS, Cavalcante WCP, Castro LHM, Nitrini R, Simabukuro MM. Updates in the Diagnosis and Treatment of Paraneoplastic Neurologic Syndromes. Curr Oncol Rep. 2018;20:92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Kang L, Wan C. Paraneoplastic syndrome in neuroophthalmology. J Neurol. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 4. | Juárez-Vignon Whaley JJ, Carrera-Muiños A, Hernandez-Gutierrez KG, Rodriguez-Cid JR, Otero-Cerdeira ME, Garcia-Montes V. Paraneoplastic Cerebellar Degeneration with Anti-CV2/CRMP5 Antibodies in Ovarian Cancer: Case Report and Review of the Literature. Case Rep Oncol. 2021;14:1799-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Lambert N, Lutteri L, Tshibanda L, Bianchi E, Maquet P. Anti-SOX1 antibody-associated acute hemorrhagic leukoencephalitis. J Neurol. 2022;269:3359-3362. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 6. | Loehrer PA, Zieger L, Simon OJ. Update on Paraneoplastic Cerebellar Degeneration. Brain Sci. 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Albadareen R, Gronseth G, Goeden M, Sharrock M, Lechtenberg C, Wang Y. Paraneoplastic autoantibody panels: sensitivity and specificity, a retrospective cohort. Int J Neurosci. 2017;127:531-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | De Simoni D, Höftberger R. [Paraneoplastic neurological syndromes : A current summary]. Internist (Berl). 2018;59:151-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Gaspard N. Autoimmune Epilepsy. Continuum (Minneap Minn). 2016;22:227-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Bucci E, Lo Muzio L, Mignogna MD. [Monocystic ameloblastoma and plexiform epithelial hyperplasia II. Clinical course and therapy]. Minerva Stomatol. 1988;37:547-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Deac S, Stana MM, Havasi AD, Cainap C, Popita AR, Bordeianu AM, Cainap S, Bota M, Bochis OV. Paraneoplastic cerebellar degeneration associated with anti-Yo antibodies in an ovarian cancer case: A case report. Gynecol Oncol Rep. 2021;35:100695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 12. | Lorusso L, Precone V, Ferrari D, Ngonga GK, Russo AG, Paolacci S, Bertelli M. Paraneoplastic Neurological Syndromes: Study of Prevalence in a Province of the Lombardy Region, Italy. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 631] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Sculier JP, Feld R, Evans WK, DeBoer G, Shepherd FA, Payne DG, Pringle JF, Yeoh JL, Quirt IC, Curtis JE. Neurologic disorders in patients with small cell lung cancer. Cancer. 1987;60:2275-2283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis. 2007;2:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Fu P, He L, Tang N, Nie Q, Li Z. A single center retrospective study of paraneoplastic neurological syndromes with positive onconeural antibodies. J Clin Neurosci. 2021;89:336-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | de Beukelaar JW, Sillevis Smitt PA. Managing paraneoplastic neurological disorders. Oncologist. 2006;11:292-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Berger B, Bischler P, Dersch R, Hottenrott T, Rauer S, Stich O. "Non-classical" paraneoplastic neurological syndromes associated with well-characterized antineuronal antibodies as compared to "classical" syndromes - More frequent than expected. J Neurol Sci. 2015;352:58-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Lowe BA, Mershon C, Mangalik A. Paraneoplastic neurological syndrome in transitional cell carcinoma of the bladder. J Urol. 1992;147:462-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | de Albóniga-Chindurza A, Riva E, Jiménez-Huete A, Graus F, Franch O. Paraneoplastic stiff person syndrome with small cell carcinoma of the bladder and anti-Ri antibodies. Clin Neurol Neurosurg. 2018;173:194-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Yi S, Park H. A rare case of aquaporin-4-antibody-positive neuromyelitis optica associated with bladder cancer. Mult Scler Relat Disord. 2020;38:101499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Zhu Y, Chen S, Song J, Chen F, Guo H, Shang Z, Wang Y, Zhou C, Shi B. An uncommon manifestation of paraneoplastic cerebellar degeneration in a patient with high grade urothelial, carcinoma with squamous differentiation: A case report and literature review. BMC Cancer. 2016;16:324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Thanarajasingam G, Milone M, Kohli M. Paraneoplastic encephalopathy: an unusual presenting feature of bladder cancer metastasis. BMJ Case Rep. 2015;2015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Prestigiacomo CJ, Balmaceda C, Dalmau J. Anti-Ri-associated paraneoplastic opsoclonus-ataxia syndrome in a man with transitional cell carcinoma. Cancer. 2001;91:1423-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Graus F, Vogrig A, Muñiz-Castrillo S, Antoine JG, Desestret V, Dubey D, Giometto B, Irani SR, Joubert B, Leypoldt F, McKeon A, Prüss H, Psimaras D, Thomas L, Titulaer MJ, Vedeler CA, Verschuuren JJ, Dalmau J, Honnorat J. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 269] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 26. | Nikolaev AY, Li M, Puskas N, Qin J, Gu W. Parc: a cytoplasmic anchor for p53. Cell. 2003;112:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Yi X, Lu H, Wu Y, Shen Y, Meng Q, Cheng J, Tang Y, Wu F, Ou R, Jiang S, Bai X, Xie K. Cystitis glandularis: A controversial premalignant lesion. Oncol Lett. 2014;8:1662-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Pantuck AJ, Bancila E, Das KM, Amenta PS, Cummings KB, Marks M, Weiss RE. Adenocarcinoma of the urachus and bladder expresses a unique colonic epithelial epitope: an immunohistochemical study. J Urol. 1997;158:1722-1727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Lukacs S, Szabo N, Woodhams S. Rare association of anti-hu antibody positive paraneoplastic neurological syndrome and transitional cell bladder carcinoma. Case Rep Urol. 2012;2012:724940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Bientinesi R, Ragonese M, Pinto F, Bassi PF, Sacco E. Paraneoplastic Dermatomyositis Associated With Panurothelial Transitional Cell Carcinoma: A Case Report and Literature Review. Clin Genitourin Cancer. 2016;14:e199-e201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Adem H, Yildirim ME, Badem S, Balçık ÖŞ. An Unusual Paraneoplastic Syndrome of Synchronous Bladder Tumor and Prostate Cancer: Polymyositis. Open J Urol. 2015;5:179-181. [DOI] [Cited in This Article: ] |
| 32. | Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler Ch, Verschuuren JJ, Vincent A, Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1122] [Cited by in F6Publishing: 1063] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 33. | Scheid R, Honnorat J, Delmont E, Urbach H, Biniek R. A new anti-neuronal antibody in a case of paraneoplastic limbic encephalitis associated with breast cancer. J Neurol Neurosurg Psychiatry. 2004;75:338-340. [PubMed] [Cited in This Article: ] |
| 34. | Vianello M, Vitaliani R, Pezzani R, Nicolao P, Betterle C, Keir G, Thompson EJ, Tavolato B, Scaravilli F, Giometto B. The spectrum of antineuronal autoantibodies in a series of neurological patients. J Neurol Sci. 2004;220:29-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |