Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11146
Peer-review started: July 1, 2022
First decision: August 1, 2022
Revised: August 9, 2022
Accepted: September 12, 2022
Article in press: September 12, 2022
Published online: October 26, 2022
With the advancement of medical technology and improvement in living standards, the incidence of multiple primary cancers has gradually increased. In particular, tumors of the digestive system account for a large proportion of multiple primary cancers. The diagnosis and treatment of chronic myeloid leukemia, particularly with synchronous gastric cancer, at the first consultation is relatively rare.
Herein, we present the case of a middle-aged man who was referred to the Department of Hematology owing to an elevated white blood cell count. After the examination, he was diagnosed with chronic myeloid leukemia and was administered imatinib. Three months after the initial diagnosis, he visited our hospital again for abdominal pain, and further examination revealed gastric malignancy. After discussion with a multidisciplinary team, S-1 (Tegafur, Gimeracil, and Oteracil Potassium Capsules) combined with oxaliplatin—SOX regimen—was initiated. Later, the patient’s condition rapidly progressed. He developed colonic obstruction and underwent an ostomy; however, he died less than 6 months after the initial diagnosis.
Multiple primary cancers are influenced by environmental and genetic factors; a standardized multidisciplinary discussion plays a key role in treatment.
Core Tip: Digestive system tumors consist of a large proportion of multiple primary cancers. In this study, we present the case of a patient with gastric cancer and concomitant chronic myeloid leukemia. To better diagnose and treat such patients, we reviewed the literature available on leukemia complicated by gastric cancer-related multiple primary cancers and analyzed its disease mechanisms, clinical symptoms, and treatment alternatives. This study will be a useful reference for the diagnosis and treatment of patients with such conditions.
- Citation: Zhao YX, Yang Z, Ma LB, Dang JY, Wang HY. Synchronous gastric cancer complicated with chronic myeloid leukemia (multiple primary cancers): A case report. World J Clin Cases 2022; 10(30): 11146-11154
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11146.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11146
Among malignant tumors, gastric cancer has the highest morbidity and mortality. Patients with early gastric cancer often exhibit no overt symptoms, whereas those with advanced gastric cancer may present with abdominal distention, discomfort, nausea, vomiting, hematemesis, and melena. In contrast, leukemia is a malignant clonal disease of hematopoietic stem cells. The primary clinical manifestations of leukemia include anemia, abnormal coagulation, fever, susceptibility to infection, hepatosplenomegaly, lymphadenopathy, and ostealgia[1]. The administration of chemotherapy drugs, such as imatinib, has resulted in a better prognosis and improved quality of life in patients with leukemia and gastric cancer[2].
Cases of patients with multiple primary cancers involving gastric cancer combined with leukemia are extremely rare, and diagnosis and treatment of this condition is difficult. Wang reported the case of an older patient with acute leukemia complicated by gastric cancer who presented with abdominal pain and discomfort but eventually died of multiple organ failure 1 mo after consultation3. This patient was initially diagnosed with chronic myeloid leukemia and then with gastric cancer 1 mo later.
Diagnosis of such patients is extremely difficult, and we reviewed the literature that could be relevant in the diagnosis and treatment of such patients.
The patient was a 43-year-old male farmer who was admitted to the hospital owing to the diagnosis of chronic myeloid leukemia for 3 mo and a 1-mo history of intermittent vomiting.
The patient showed an elevated white blood cell count during the national grassroots public welfare physical examination at a local hospital 3 mo before admission. He did not report fever, chills, low-grade fever, night sweats, dizziness, headache, or rash. Next, he was referred to a higher-level hospital for a more thorough blood examination, which revealed the following results: red blood cell count, 3.57 × 1012/L; white blood cell count, 150.27 × 109/L; promyelocyte percentage, 15%; mature lymphocyte percentage, 60%; and mature monocyte percentage, 15%. Later, inpatient examination revealed the following results: white blood cell count, 136.59 × 109/L, red blood cell count, 3.82 × 1012/L; neutrophil percentage, 90.7%; and neutrophil count, 123.98 × 109/L. T and B lymphocyte immune function test revealed the following findings: cluster of differentiation (CD)3+, 31.2%; CD3+ and CD4+, 15.7%; CD3+ and CD8+, 12.0%; CD4+/CD8+, 1.31%; and CD3− and CD19+, 4.5%. The bone marrow cytology test revealed the following findings: (1) Myeloid hyperplasia was observed to a significant extent with 93.5% myeloid cells and 6.5% erythroid cells; (2) The granule:red ratio was 14.38:1; (3) The myeloid system was abnormally hyperplastic, particularly with middle and late myelocytes; eosinophils, basophils, and mitotic cells were also observed; (4) The extent of erythroid hyperplasia was relatively low; (5) A decreased proportion of lymphocytes was observed; and (6) Seven megakaryocytes, which were granular giant cells, were observed within 1 wk.
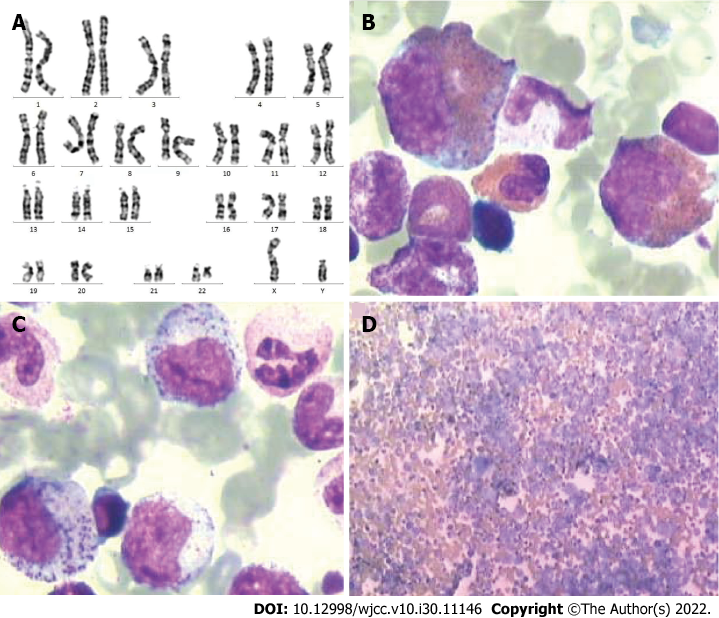
Identifying middle and late myelocytes on blood films was easy. The size of the red blood cells did not change, the bone marrow smear filling was acceptable, and platelets were scattered and clustered. At this stage, chronic myeloid leukemia was considered. The leukemia immunophenotyping test revealed the following results: A blast cell area accounting for approximately 0.6% of nuclear cells was observed; increased proportion of granulocytes by approximately 90.7% was noted; fully expressed cMPO, CD13, CD33, and CD64 along with partially expressed CD16, CD15, CD11b, CD10, and CD4 were observed, and the remaining were negative. An early myeloid development pattern was observed, with most development observed in the middle stage; therefore, mature granulocyte proliferative disease (chronic phase chronic myeloid leukemia) was considered. The level of P170 (CD243) was 0.2%. The digital quantitative analysis test detected the BCR-ABL-p210 fusion gene. Chest computed tomography (CT) scan revealed the following results: (1) Micronodules were observed in the posterior segment of the left upper lobe apex, which could be old lesions; and (2) The local bone of the left fifth anterior rib was rough and dense, which could be an old fracture. Color Doppler showed slight tricuspid valve and aortic regurgitation but normal left ventricular systolic and diastolic function.
At this point, the diagnosis of chronic myeloid leukemia was clear (Figure 1), and symptomatic treatment (alkalization, hydration, and plasma cells) was provided. After the white blood cell count reduced to a safe level, he was treated with imatinib mesylate and discharged. One month after discharge, the patient developed pain in the left lower extremity, which was not reported in the consultation. Later, he developed nausea, which was relieved after vomiting a small amount of gastric content. This was accompanied by a productive cough with white sticky sputum, but he did not show fever, chills, abdominal pain, or diarrhea. His body weight decreased by 7.5 kg over 3 mo.
The patient was a healthy individual without diabetes, hypertension, or other underlying diseases.
The patient had no contributory family history.
Physical examination showed slight abdominal tenderness, with no rebound tenderness and tension.
For further diagnosis and treatment, we performed a series of laboratory and imaging examinations. The results of the blood routine and biochemical tests were normal. The four tests for hematopoiesis showed a slight decrease in vitamin B12 and ferritin levels.
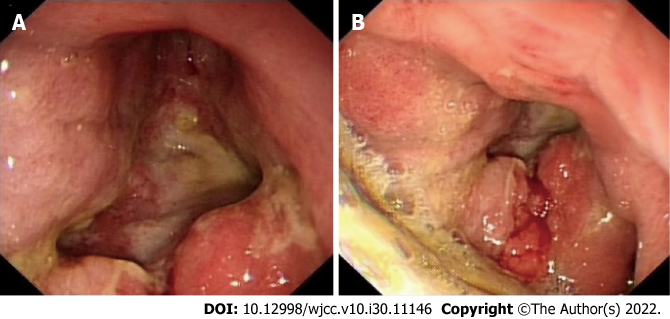
CT examination of the chest and abdomen showed an increase in the thickness of the wall of the gastric antrum and pylorus. It revealed a blurred surrounding fat space and the presence of multiple enlarged lymph nodes in the cardia, hepatic hilum, greater omentum, and retroperitoneum (Figure 2). A whole body bone scan revealed abnormal bone metabolism in the bilateral scapula, rib, and iliac bone; thus, multiple bone metastases were considered (Figure 3). Meanwhile, gastroscopy showed the presence of a large amount of retained material in the gastric cavity; yellowish mucus; and huge ulcers in the lower part of the gastric body, gastric angle, and gastric antrum. Although the pylorus was deformed, the lens body could pass through it. These findings were suggestive of gastric cancer (Borrmann type IV) with bile reflux 2 and reflux esophagitis grade C (Figure 4).
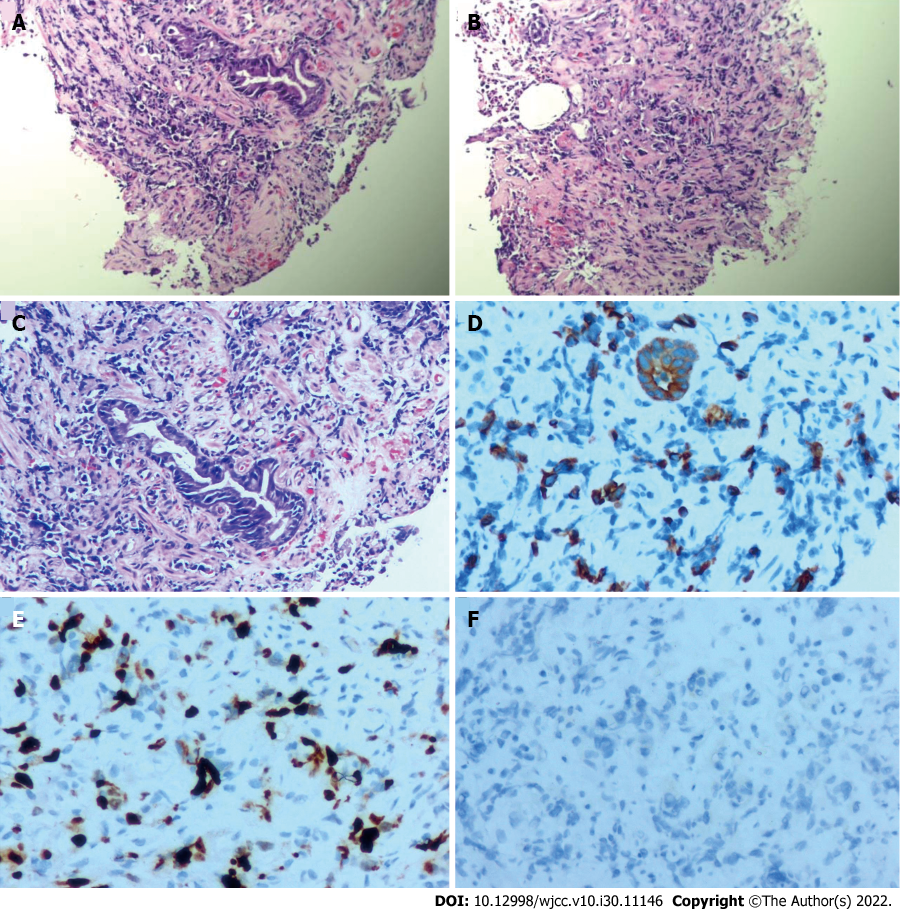
Pathological analysis revealed a small number of atypical cells in the gastric antrum. Immunohistochemical results were as follows: CKL(2+), CD56(−), ki67 (40%), CD68 (focal 1+), her-2(1+), MPO(−), CD117(−), CD15 (focal 1+), and CD34 (vascular+). Considering the results of morphological and immunohistochemical analyses (Figure 5), poorly differentiated adenocarcinoma was diagnosed.
Based on the patient’s medical history, clinical characteristics, and diagnostic results, the final diagnosis was advanced gastric cancer, abdominal lymph node metastasis, bone metastasis, and chronic myeloid leukemia.
Owing to the distinct condition of the patient, we opted to conduct a multidisciplinary discussion on this case. It was decided that imatinib (400 mg, once daily) administration was to be continued for chronic myeloid leukemia and SOX regimen to be started for gastric cancer with bone metastasis. Antiemetics and acid-suppressing drugs were used as adjuvant therapy.
The patient had severe nausea and vomiting during the second cycle of chemotherapy, which was attributed to incomplete intestinal obstruction (Figure 6). However, the obstruction was not relieved after conservative treatment, and exploratory laparotomy and ileostomy were performed.
Following laparotomy, we found severe adhesion of the gastric antrum and colonic hepatic flexure. Portions of the small intestine, ascending colon, and transverse colon showed considerable dilatation. Multiple white, hard nodules were observed in the small mesentery. Considering the preoperative examination results, we decided to perform terminal ileal double-lumen fistula. An area of 1 cm around the right McBurney’s point was considered the stoma. The skin and subcutaneous tissue were incised layer-by-layer, and the terminal double ileum was led out through the ileocecal region, 15 cm away from the ileocecal opening. A double-lumen stoma was performed, and the stoma was sutured layer-by-layer for reinforcement.
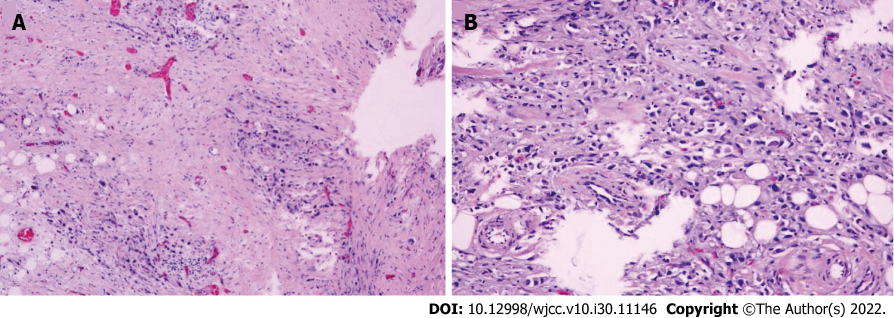
During the operation, several hard nodules were harvested for subsequent biopsy, which revealed metastatic adenocarcinoma (Figure 7). After discharge, the patient unfortunately passed away in December 2021 (less than 6 mo since the first consultation).
The continuous progress in medicine and improvement in living standards have extended the lifespan of humans. In addition, the detection rate of multiple primary cancers has gradually increased[4].
Duplicate carcinoma was first reported by Billroth in 1879. Also referred to as multiple primary malignant tumors, duplicate carcinoma involves the simultaneous or successive occurrence of two or more different pathological tissue types in the same organ or tissue or in different organs or tissues in different parts of the same individual. Briefly, it is the presence of two or more primary malignancies. According to the time of appearance of the second malignant tumor, multiple primary cancers can be categorized into synchronous multiple primary cancers (interval of < 6 mo) and metachronous multiple primary cancers (interval ≥ 6 mo). The diagnostic criteria for multiple primary cancers are as follows: (1) Each tumor is malignant; (2) Each tumor occurs in different parts and exists independent of each other; (3) Each tumor has unique morphological characteristics and its own metastatic pathway; and (4) Tumors showing recurrence and metastasis are excluded[5].
There are several pathogenic mechanisms underlying multiple primary cancers, and the most studied mechanisms are those underlying the carcinogenic effects of radiotherapy and chemotherapy, environmental factors, genetic factors, and gene interactions. A previous study reported the case of a woman who underwent total gastrectomy for advanced gastric cancer and received regular postoperative TS-1 chemotherapy; she was eventually diagnosed with secondary chronic myeloid leukemia after 3 years[6]. Another older male patient underwent gastric cancer surgery and was diagnosed with chronic myeloid leukemia 2 years after TS-1 chemotherapy[7]. Furthermore, chronic myeloid leukemia has been reported after chemotherapy using drugs other than TS-1[8]. Moreover, postoperative follow-up studies have shown that patients with breast cancer who received radiotherapy and chemotherapy after surgery have a substantially higher chance of developing multiple primary cancers[9]. Furthermore, environmental factors, such as long-term exposure to chemical and radioactive substances, may be associated with the occurrence of multiple primary cancers[10]. The incidence and prognosis of multiple primary cancers vary among different ethnic groups, which might be because of the effects of genetic factors[11]. A retrospective analysis of cervical cancer and endometrial cancer showed that radiation therapy, smoking, and human papillomavirus infection were significantly associated with the risk of multiple primary cancers[12]. Moreover, frequent exposure to radioactive substances, especially I131 therapy, increased the risk of second primary cancer after thyroid cancer surgery. Because of the limited number of cases of multiple primary cancers and the lack of research at present, the mechanism underlying gene interaction in the occurrence of multiple primary cancers remains unelucidated.
The patient in this case was a middle-aged man from a rural area who had no bad living habits. He had an acute onset and rapid disease progression, which may be attributed to multiple factors. First, his nutritional status was poor, with reduced immune surveillance capacity. Second, there may be a genetic interaction between the first primary cancer leukemia and gastric cancer. In particular, leukemia inhibitory factor and myeloid leukemia-1 are differentially expressed in the tissues of patients with leukemia and gastric cancer, and both play key roles in disease development. However, further studies on the interaction mechanism are warranted[13-16]. Third, during the treatment for leukemia with imatinib, the patient showed severe adverse gastrointestinal reactions, which necessitated short-term drug suspension (4 d) treatment, thereby resulting in the rapid progress of gastric cancer.
In patients with leukemia and gastric cancer, the clinical symptoms associated with leukemia and gastric cancer can either appear simultaneously or successively. The diagnosis is primarily based on blood routine, bone marrow biopsy, imaging, gastroscopy, and pathological analyses. In terms of treatment, acute leukemia is preferentially treated when it is associated with gastric cancer. When gastric cancer is complicated by bleeding, obstruction, and other emergencies, prioritizing the treatment of gastric cancer is essential. However, the effect of imatinib in the development of chronic myeloid leukemia secondary to gastric cancer chemotherapy should be noted. In cases of gastric cancer complicated by leukemia, a multidisciplinary discussion on several fields, including radiology, pathology, gastroenterology, hematology, radiotherapy, and general surgery, is ideal for formulating the best treatment plan for the patient[17,18].
Metachronous duplication carcinoma has a significantly better survival time and prognosis compared with synchronous duplication carcinoma owing to early detection of the second primary cancer of metachronous duplication carcinoma[19]. Existing studies have reported that early diagnosis and treatment can substantially improve the survival and prognosis of patients with multiple primary cancers.
This patient had abdominal discomfort at the initial diagnosis and underwent abdominal B-ultrasound examination; however, gastric cancer was not detected. Advanced gastric cancer was detected on CT conducted after 1 mo of abdominal pain and discomfort. In such patients, routine chest and abdominal plain CT examinations at the initial diagnosis can be useful in the timely detection of multiple primary cancers. In addition, for further diagnosis and treatment of such patients, particular gastric cancer types—such as hepatoid adenocarcinoma of the stomach, lymphoepithelioma-like gastric carcinoma, and hereditary diffuse gastric cancer (HDGC)—cannot be ignored. Tumor markers, gastrin, C14 urea breath testing, CHD1 detection, and colonoscopy are useful in the diagnosis and treatment of these patients[20-22].
Despite the rapid progress in medical technology, more studies should be conducted for the timely diagnosis and treatment of patients with gastric cancer complicated by chronic myeloid leukemia. There is an urgent need to strengthen the understanding of recurrent cancer. For patients with high-risk factors for multiple primary cancers, conducting more comprehensive examinations, formulating an appropriate diagnosis, and ensuring adequate treatment and follow-up are critical. Appropriate guidelines regarding the diagnosis and treatment of recurrent cancer are required in addition to multidisciplinary discussions to formulate optimal treatment plans for patients with multiple primary cancers. Simultaneous gastric cancer with chronic myeloid leukemia has a worse prognosis and shorter survival time than its metachronous counterpart. The pathogenesis of gastric cancer complicated by chronic myeloid leukemia should be further studied because of the current challenges in the diagnosis and treatment of patients with malignant tumors.
We wish to thank all the medical workers involved in the diagnosis and treatment of this patient, especially the multidisciplinary team of the First Hospital of Lanzhou University for their guidance in the diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Hakimi T, Afghanistan; Sumi K, Japan; Yakar M, Turkey S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Bosch F, Dalla-Favera R. Chronic lymphocytic leukaemia: from genetics to treatment. Nat Rev Clin Oncol. 2019;16:684-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8287] [Cited by in F6Publishing: 10162] [Article Influence: 3387.3] [Reference Citation Analysis (2)] |
| 3. | Dongchu W, Rong Z. A case of gastric cancer with acute leukemia synchronous duplication carcinoma. Hainan Medical Journal. 2018;29:1615-1616. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Zheng X, Li X, Wang M, Shen J, Sisti G, He Z, Huang J, Li YM, Wu A; Multidisciplinary Oncology Research Collaborative Group (MORCG). Second primary malignancies among cancer patients. Ann Transl Med. 2020;8:638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Copur MS, Manapuram S. Multiple Primary Tumors Over a Lifetime. Oncology. 2019;33. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Higuchi M, Nishinak H, Yamano Y. Differences in Acetic Acid Ototoxicity in Guinea Pigs are Dependent on Maturity. Otolaryngol (Sunnyvale). 2015;5:208. [DOI] [Cited in This Article: ] |
| 7. | Miyati T, Imai H, Ogura A, Doi T, Tsuchihashi T, Machida Y. Novel SNR determination method in parallel MRI. Proc. SPIE 6142, Medical Imaging 2006: Physics of Medical Imaging, 61423O [cited 2 March 2006]. [DOI] [Cited in This Article: ] |
| 8. | Tsuzuki M, Handa K, Yamamoto K, Hasegawa A, Yamamoto Y, Watanabe M, Mizuta S, Maruyama F, Okamoto M, Emi N, Ezaki K. Chronic myeloid leukemia following chemotherapy with 5'-deoxy-5-fluorouridine for gastric cancer. Intern Med. 2008;47:1739-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Jabagi MJ, Goncalves A, Vey N, Le Tri T, Zureik M, Dray-Spira R. Risk of Hematologic Malignant Neoplasms after Postoperative Treatment of Breast Cancer. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Nosrati A, Yu WY, McGuire J, Griffin A, de Souza JR, Singh R, Linos E, Chren MM, Grimes B, Jewell NP, Wei ML. Outcomes and Risk Factors in Patients with Multiple Primary Melanomas. J Invest Dermatol. 2019;139:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Sidhom F, Jackson D, Ali A, Shokrani B, Taddesse-Heath L. Multiple Primary Malignant Neoplasms in African Americans: A Case Series and Literature Review. Cureus. 2022;14:e21585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Huang K, Xu L, Jia M, Liu W, Wang S, Han J, Li Y, Song Q, Fu Z. Second primary malignancies in cervical cancer and endometrial cancer survivors: a population-based analysis. Aging (Albany NY). 2022;14:3836-3855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 13. | Xu Z, Chen J, Shao L, Ma W, Xu D. Promyelocytic leukemia protein enhances apoptosis of gastric cancer cells through Yes-associated protein. Tumour Biol. 2015;36:8047-8054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Xu G, Wang H, Li W, Xue Z, Luo Q. Leukemia inhibitory factor inhibits the proliferation of gastric cancer by inducing G1-phase arrest. J Cell Physiol. 2019;234:3613-3620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Likui W, Qun L, Wanqing Z, Haifeng S, Fangqiu L, Xiaojun L. Prognostic role of myeloid cell leukemia-1 protein (Mcl-1) expression in human gastric cancer. J Surg Oncol. 2009;100:396-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Seeneevassen L, Martin OCB, Lehours P, Dubus P, Varon C. Leukaemia inhibitory factor in gastric cancer: friend or foe? Gastric Cancer. 2022;25:299-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Carlson RW, Anderson BO, Burstein HJ, Carter WB, Edge SB, Farrar WB, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Jahanzeb M, Ljung BM, Kiel K, Marks LB, McCormick B, Nabell LM, Pierce LJ, Reed EC, Silver SM, Smith ML, Somlo G, Theriault RL, Ward JH, Winer EP, Wolff AC. Invasive breast cancer. J Natl Compr Canc Netw. 2007;5:246-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Sharma A, Sethi N, Saini S, Pandia K, Jangir R. Primary Ewings sarcoma in liver - A rare case report with review of literature. Indian J Pathol Microbiol. 2021;64:S136-S139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Ochiai K, Kawai K, Nozawa H, Sasaki K, Kaneko M, Murono K, Emoto S, Ishii H, Sonoda H, Yamauchi S, Sugihara K, Ishihara S. Prognostic Impact and Clinicopathological Features of Multiple Colorectal Cancers and Extracolorectal Malignancies: A Nationwide Retrospective Study. Digestion. 2021;102:911-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Guilford P, Humar B, Blair V. Hereditary diffuse gastric cancer: translation of CDH1 germline mutations into clinical practice. Gastric Cancer. 2010;13:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Yu C. Comment on: "Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer, 2019: 1-10" by Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, Yuan J, Wang X, Li J, Lu Z, Gong J, Lu M, Zhou J, Peng Z, Shen L, Zhang X. Gastric Cancer. 2019;22:1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Guimaraes N, Bolais Monica I, Oliveira S, Pato Pais D, Andrade S, Bertao Colaco I, Vila Nova C, Vieira V, Azenha N, Fonseca Pinho J, Guedes F, Conceicao L, Valente Cecilio J. Lymphoepithelioma-Like Gastric Carcinoma and Gastric Involvement of Chronic Lymphocytic Leukemia: Two Rare Identities Coexisting in One Patient. J Med Cases. 2022;13:36-39. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |