Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.8025
Peer-review started: February 20, 2022
First decision: March 24, 2022
Revised: April 4, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: August 6, 2022
Carotid artery pseudoaneurysm (PSA) is infrequently encountered in clinical settings. Internal carotid artery (ICA) PSA complicated with ischemic stroke is rare. PSAs are typically caused by iatrogenic injury, trauma, or infection. The underlying mechanisms of spontaneous PSA formation are not well characterized. We report a healthy young man who presented with stroke as a complication of spontaneous PSA of the left ICA.
A 30-year-old man working as a ceiling decoration worker was hospitalized due to sudden-onset speech disorder and right lower extremity weakness. Medical history was unremarkable. Brain computed tomography revealed ischemic stroke. Digital subtraction angiography showed a left ICA PSA with mild stenosis. The patient was conservatively managed with oral anticoagulation and antiplatelet therapy. He recovered well and was discharged. The patient was in good condition during follow-up.
The occupational history of patient should be taken into consideration while evaluating the etiology of spontaneous ICA PSA in young people with stroke.
Core Tip: In a previously healthy youngster with stroke, it is counterintuitive to make a connection between stroke and pseudoaneurysm (PSA), especially if there is no obvious cause. To best of our knowledge, this is the first report of spontaneous carotid artery PSA with stroke in a young adult. This case report may provide insights for diagnosis of carotid artery PSA in youngsters. Conservative therapy is a viable alternative for young patients with small carotid PSA.
- Citation: Zhong YL, Feng JP, Luo H, Gong XH, Wei ZH. Spontaneous internal carotid artery pseudoaneurysm complicated with ischemic stroke in a young man: A case report and review of literature. World J Clin Cases 2022; 10(22): 8025-8033
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/8025.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.8025
Arterial wall has a three-layered structure comprising of intima, media, and adventitia[1]. Rupture of the arterial wall may occur due to several reasons, such as iatrogenic injury, trauma, infection, or tumor invasion[2]. Disruption of the arterial wall following injury leads to formation of hematoma adjacent to the artery; subsequent proliferation of peripheral fibroblasts may result in encapsulation and organization of the hematoma leading to the formation of pseudoaneurysm (PSA)[3]. A previous study has shown that PSA formation is the most common complication of endovascular intervention with the incidence rates ranging from 0.7% to 6.25%. Femoral arteries and cardiovascular is the most common site of formation of PSA[4]. Traumatic internal carotid artery (ICA) PSA is a rare entity, with an incidence of approximately 9% in cases with head and neck trauma[5]. The clinical manifestations depend on the size, site, and etiology of the PSAs; however, the development of PSA can cause severe complications such as rupture, stroke, or asphyxia[6,7]. Digital subtraction angiography (DSA) has a high sensitivity and specificity for the diagnosis of ICA PSA and is considered as the diagnostic gold standard of PSA[8].
In this case report, we describe a case of a 30-year-old male who suffered speech disorders and right lower extremity weakness and review the previously reported cases.
A 30-year-old man was admitted to the Neurology department of our hospital because of the chief complaints of speech disorder and right lower extremity weakness five days ago.
Five days ago, the patient developed sudden-onset speech disorder and right lower extremity weakness at work and was admitted to a local hospital. The condition of the patient showed gradual improvement after administration of thrombolytic treatment. The etiology of stroke was still unknown. In order to seek more comprehensive diagnosis and treatment, the patient was referred to the Neurology department of our hospital.
The patient had no history of hypertension, diabetes, or coronary artery disease. Furthermore, there was no history of acute trauma or iatrogenic injury.
The patient was a ceiling decoration worker. He had no history of smoking and alcohol consumption. Personal and family history was unremarkable. There was no family history of connective tissue disease, such as Marfan syndrome.
On physical examination, the patient was found to have a hemiparetic gait. The muscle strength of right upper and lower limbs was grade 4 and the light touch sensation was attenuated on the right side. Babinski sign was found in the sole of his right foot. Other physical findings were unremarkable.
Routine blood parameters were as follows: Leukocyte count 11.37 × 109/L; platelet count 344 × 109/L; neutrophils 84.9%; plasma fibrinogen 4.12 g/L; lactic dehydrogenase 287 U/L. Renal function and liver function tests were normal.
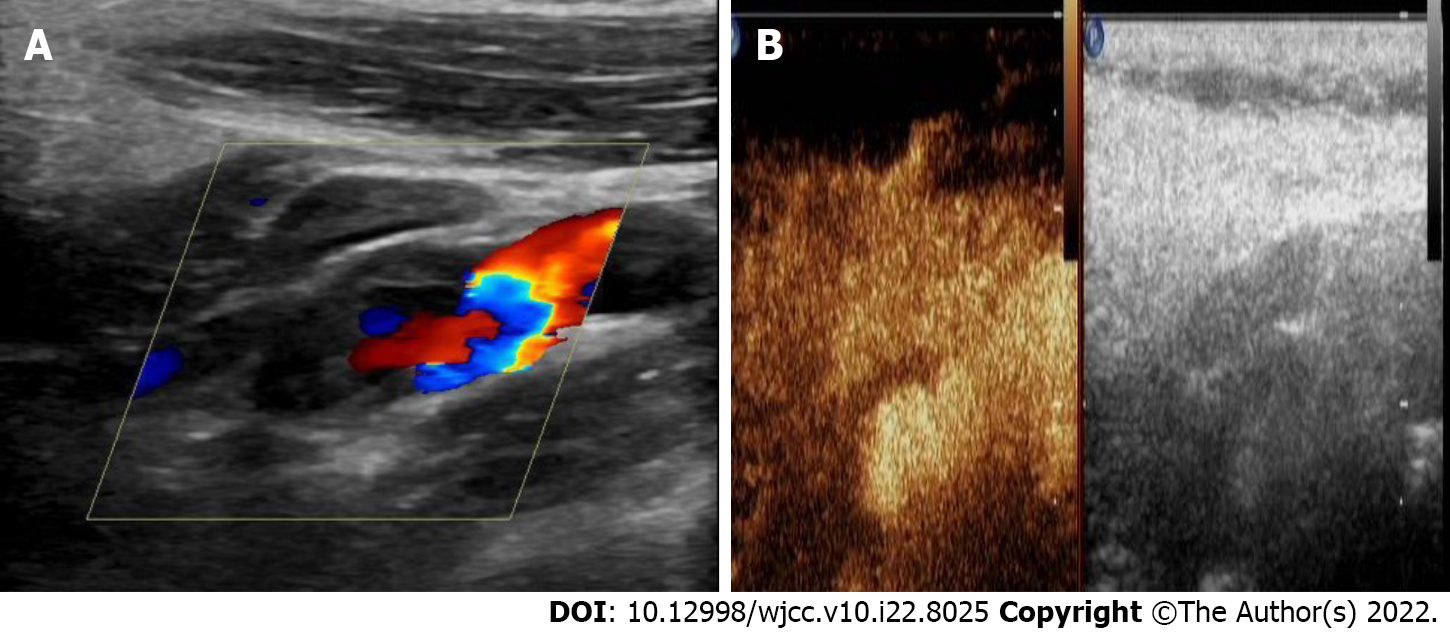
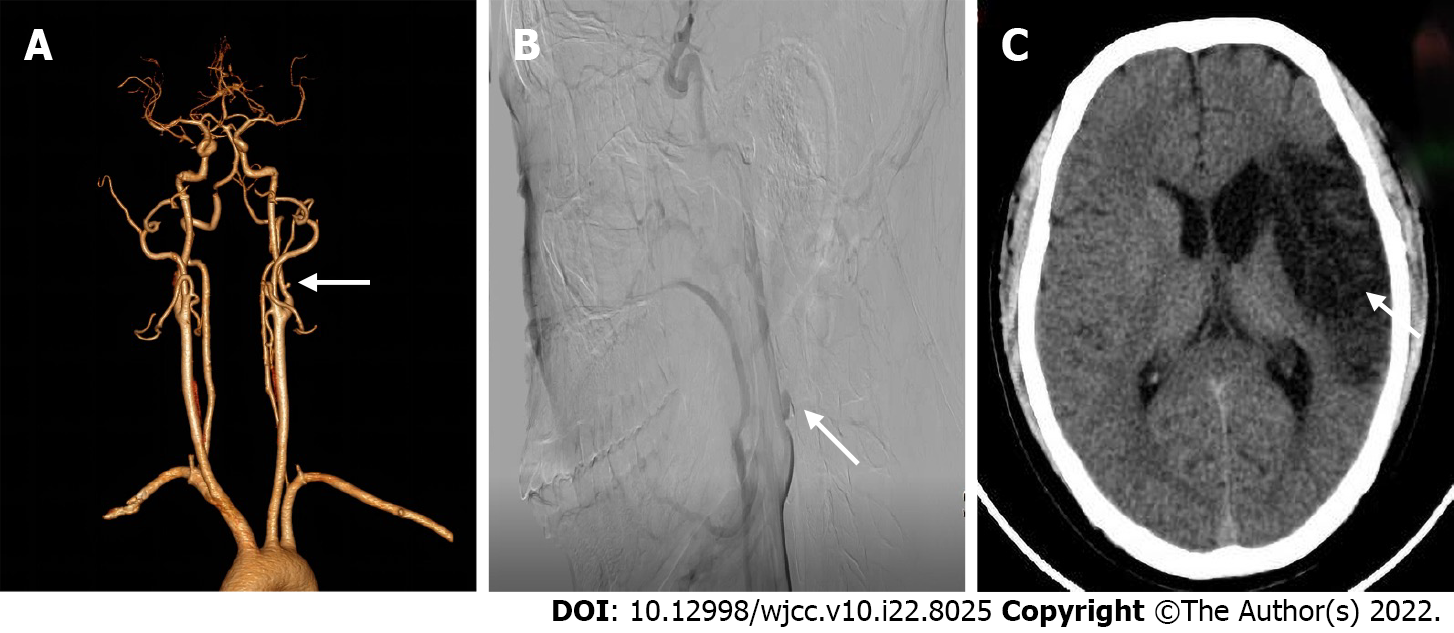
Cerebral computed tomography showed low-density foci in the left frontotemporal and centroparietal regions, which were indicative of left ischemic stroke. Ultrasonography of the carotid arteries exhibited a mixed echogenic mass at the origin of the left ICA (Figure 1). Computed tomography angiography (CTA) revealed a nodular mass with mural thrombus in continuity with the adjacent left ICA lumen; the size of the mass was approximately 10 mm × 7 mm (Figure 2). DSA indicated a PSA at the origin of the left ICA with mild stenosis.
Left ICA PSA complicated with ischemic stroke.
Low-dose alteplase and oral anticoagulation and antiplatelet therapy. For ischemic stroke, the local hospital evaluated the condition of patient and opted for low-dose alteplase to maintain the benefits of treatment while reducing the risk of systemic or intracerebral hemorrhage[9]. As for carotid PSA, the patient failed the ICA temporary occlusion test, which implied that his cerebral arteries could not develop sufficient cerebral collateral circulation. Therefore, we intended to use a combination of endovascular stent placement and coil embolization to treat the PSA. However, the patient refused this treatment option because of the costs and the associated risks. Therefore, he was conservatively managed with oral anticoagulation and antiplatelet therapy.
The patient recovered satisfactorily and was discharged from hospital on day 8. In order to prevent recurrence of ischemic stroke, he was prescribed oral aspirin for one month[9]. The patient was found to be in a good condition on follow-up evaluation performed at 3 and 6 months. Cerebral computed tomography (CTA) showed a large encephalomalacia focus in the left temporal-basal region, which indicated that the patient was at the convalescent stage of ischemic stroke. On cervical CTA, the size of PSA at the origin of the left carotid artery was significantly smaller than before, which was consistent with the results of DSA (Figure 3). However, we did not obtain further follow-up data for patients beyond 6 months.
The incidence of stroke in young people has increased over the past decades, reaching 221 per 100000 by the end of 2019. Underlying cardiovascular disease is the main cause of stroke in this population, while aneurysms or PSA are not common causes of stroke[10,11]. PSA typically occurs due to iatrogenic injury, trauma, infection, and tumor invasion[3,12]. Spontaneous PSA are rare entities. Spontaneous PSA associated with stroke are exceedingly rare[13]. A comprehensive search of the literature was performed using the PubMed, Embase, Cochran Library and Web of Science databases to retrieve studies published before December 2021 (Supplementary material). In the 16 cases reviewed by us, the etiology of 5 cases (31%) was trauma[14-18] and 4 cases (25%) had iatrogenic injury[19-22], while only 2 cases (13%) were spontaneous; however, both spontaneous cases had a history of hypertension and hyperlipidemia[23,24] (Table 1). Our patient was a young adult with no personal or family history of cardiovascular disease. Moreover, there was no history of neck trauma or surgery on the neck. We speculated that the etiology was related to the nature of the patient's job. The patient worked as a ceiling decorator, whose daily work entailed prolonged extension of the neck for working on the ceiling. The prolonged neck extension may have caused damage to the wall of the ICA, which contributed to the formation of PSA. Moreover, PSAs are more prone to thrombosis due to vortex in the PSAs[25]. The patient developed sudden weakness of the right lower limb and speech disorder at work, which may be due to the hemodynamic changes at the thrombus site caused by the change in head posture. Subsequently, the thrombus embolized to the M1 segment of the left middle cerebral artery, resulting in ischemic stroke of the temporoparietal lobe[26]. Our experience suggests that carotid PSA should be considered when evaluating a patient presenting with stroke. what’s more, it is necessary to perfect the relevant examinations.
| Case No. | Ref. | Year | Age | Sex | Site | Etiology | Imaging modality | Treatments | Outcome | Follow-up |
| 1 | [14] | 2000 | 19 | M | R CCA | Traumatic | CT, US, MRA, DSA | Surgical resection | REC | NA |
| 2 | [35] | 2005 | 62 | M | L INA | Infectious | US, MRI | Surgical resection | REC | 1 mo |
| 3 | [19] | 2007 | 60 | M | R ICA | Iatrogenic | MRI, DSA | Endovascular stenting | REC | NA |
| 4 | [15] | 2007 | 31 | M | R ICA | Traumatic | MRI, CT, DSA | Endovascular stenting | REC | NA |
| 5 | [24] | 2008 | 51 | M | L ICA | Spontaneous | MRI, CT, DSA | Endovascular stenting | REC | NA |
| 6 | [43] | 2014 | 71 | M | L ICA | Radioactive | US, CT, MRI, DSA | Endovascular occlusion | REC | NA |
| 7 | [16] | 2013 | 46 | M | L ICA | Traumatic | CT, DSA | Endovascular stenting | REC | 14 mo |
| 8 | [44] | 2017 | 2 | M | L ICA | Infectious | MRI, CT, DSA | Endovascular occlusion | REC | 1 mo |
| 9 | [23] | 2018 | 85 | M | L ECA | Spontaneous | US, CT, MRI, DSA | Conservative therapy | Death | 7 mo |
| 10 | [45] | 2019 | 60 | F | L ECA | Congenital | MRI, CT, DSA | Endovascular stenting | REC | NA |
| 11 | [22] | 2019 | 45 | M | L CCA | Iatrogenic | CT, CTA | NA | REC | NA |
| 12 | [20] | 2019 | 57 | F | L CCA | Iatrogenic | CT, CTA | Endovascular occlusion | REC | 6 mo |
| 13 | [21] | 2019 | 79 | M | R FA | Iatrogenic | CT, MRI, CTA | Endovascular stenting | NA | NA |
| 14 | [46] | 2020 | 53 | M | SA | Infectious | CT, DSA | Conservative therapy | REC | NA |
| 15 | [17] | 2020 | 31 | F | L VA | Traumatic | CT, CTA, DSA | Conservative therapy | REC | 6 mo |
| 16 | [18] | 2021 | 35 | M | L ICA | Traumatic | US, MRI, MRA | Endovascular occlusion | REC | 3 mo |
| Present case | 2022 | 30 | M | L ICA | Spontaneous | US, CT, CTA, DSA | Conservative therapy | REC | 6 mo |
Carotid ultrasonography, a noninvasive, cost-effective, and radiation-free method, is currently the first-line imaging modality for screening carotid artery PSA. Doppler sonography can help distinguish a PSA from an aneurysm and/or other cervical mass[27]. It typically shows a neck mass with the typical features of PSA, including spontaneously echogenic swirling flow in the lumen and “to-and-fro” waveforms at the neck[28]. However, the “to-and-fro” waveforms were not observed in our patient, probably because of the relatively small tumor size. Furthermore, it is difficult to directly detect an ICA PSA that is located about 20 mm above the bifurcation of the common carotid artery[29]. In our patient, although this PSA was located 17 mm above the common carotid artery bifurcation, when we found a mass in the initial part of the ICA, we performed contrast-enhanced ultrasound (CEUS) of the carotid artery. CEUS showed contrast agent filling in the distended area of the left ICA, but no enhancement in the low echo area of the mural (Video 1). This finding suggests that the combination of ultrasonography and CEUS of the carotid artery may facilitate the diagnosis of PSA located at a relatively high position. CTA can effectively depict the localization, size, and mural thrombus of PSA[30]; furthermore, CTA with 3D reconstruction maps can delineate the outer wall of PSA and its relationship with peri-PSA vascular structures, which can provide surgeons with intuitive 3D image guidance[8]. In our case, CTA reconstruction revealed a nodular mass with mural thrombus in continuity with the adjacent left ICA lumen, which was an important anatomical information. The gold standard for the diagnosis of PSA is DSA with > 99% sensitivity and 100% specificity[8,31]. Out of the 16 reported cases, DSA was used as a diagnostic method in 11 cases (69%). In the present case, angiography showed the contrast agent entering the tumor cavity along with changes in eddy currents, which indicated rupture of the left ICA wall and the formation of PSA. Furthermore, the parent artery was localized with delayed distal development, which indicated compression of ICA. Although the diagnostic performance of DSA is pretty good, it is difficult to detect PSA that is filled with thrombus at the early stage[32].
Surgery and endovascular therapy are two main treatment modalities for carotid PSA[33]. Because of the severe complications of surgery and the rapid advances in the field of endovascular intervention, endovascular therapy has emerged as the preferred treatment for carotid PSA, especially for patients with PSA who present with stroke[34]. Surgical resection is used as an alternative to endovascular treatment. In addition, the choice of endovascular therapy depends on the lesion site and the performance status of patient[14]. Out of the 16 reviewed cases, only 3 patients (13%) were treated with surgical resection[14,35]. Endovascular therapy mainly includes use of covered stent grafts, micro-coil embolization, and detachable balloon embolization[36]. Choice of endovascular treatment depends on multiple factors, mainly the site of PSA, age of patient, and intracranial collateral circulation[37]. ICA temporary occlusion test should be performed first for PSAs occurring in the extracranial ICA[38]. If the test is successful, the ICA can be permanently occluded using a detachable balloon. If the test fails, the patient can be treated with covered stent grafts and accessory micro-coil embolization[39]. Our patient failed the ICA temporary occlusion test, which indicated the lack of adequate cerebral collateral circulation. Therefore, we intended to combine endovascular stent placement and coil embolization to treat the PSA; however, the patient opted for conservative management owing to the high cost of treatment and the associated risks. At 6-mo follow-up, the patient was in a relatively good condition and cervical CTA showed significant reduction in the size of PSA. Anticoagulant and antiplatelet agents may decrease mortality related to carotid PSA; however, such conservative management alone is not recommended owing to the risk of delayed rupture of PSA of the carotid artery, which is a life-threatening condition[40]. Out of the 16 cases reviewed, only 3 patients (19%) were conservatively managed. Budincevic et al[25] reported an 85-year-old man who died after receiving conservative therapy. However, Xue et al[17] reported a 31-year-old woman who showed satisfactory outcome with conservative treatment, which is consistent with our present case. Our patient may have shown better efficacy of conservative treatment owing to the relatively small size of the aneurysm. In addition, previous studies have shown that the choice of endovascular therapy should depend on the etiology of PSAs and that endovascular therapy is not necessary for all types of PSAs[41,42]. Thus, it is important to select appropriate treatment according to the etiology. Our report may provide an alternative therapy for young patients with small carotid PSA, nevertheless, the length of follow-up in our report was relatively short, which is its limitation.
We report a young man with clinical presentation of ischemic stroke that was triggered by thrombosis of PSA. The etiology of spontaneous ICA PSA in this case remains unknown. We inferred that the etiology may be related to the characteristics of the patient's occupation. Therefore, history of trauma, infection, and occupational history should be carefully elicited in young patients with acute ischemic stroke who have no history of cardiovascular disease. DSA is the gold standard for the diagnosis of carotid PSA; however, the combination of CEUS and conventional ultrasonography of the carotid artery may facilitate the diagnosis of PSA that is located at a relatively high position. Last but not least, although endovascular therapy is the recommended treatment for carotid PSA, the treatment strategy should be personalized based on the patient characteristics. Conservative therapy may be a viable alternative for young patients with small carotid PSA.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soliman MA, Egypt; Umana GE; Velnar T, Slovenia; Yap RVC, Philippines S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Nadig S, Barnwell S, Wax MK. Pseudoaneurysm of the external carotid artery--review of literature. Head Neck. 2009;31:136-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Béjot Y, Daubail B, Debette S, Durier J, Giroud M. Incidence and outcome of cerebrovascular events related to cervical artery dissection: the Dijon Stroke Registry. Int J Stroke. 2014;9:879-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Mahmoud MZ, Al-Saadi M, Abuderman A, Alzimami KS, Alkhorayef M, Almagli B, Sulieman A. "To-and-fro" waveform in the diagnosis of arterial pseudoaneurysms. World J Radiol. 2015;7:89-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (2)] |
| 4. | Oweida SW, Roubin GS, Smith RB 3rd, Salam AA. Postcatheterization vascular complications associated with percutaneous transluminal coronary angioplasty. J Vasc Surg. 1990;12:310-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 125] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Cox MW, Whittaker DR, Martinez C, Fox CJ, Feuerstein IM, Gillespie DL. Traumatic pseudoaneurysms of the head and neck: early endovascular intervention. J Vasc Surg. 2007;46:1227-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Fankhauser GT, Stone WM, Fowl RJ, O'Donnell ME, Bower TC, Meyer FB, Money SR. Surgical and medical management of extracranial carotid artery aneurysms. J Vasc Surg. 2015;61:389-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Li Z, Chang G, Yao C, Guo L, Liu Y, Wang M, Liu D, Wang S. Endovascular stenting of extracranial carotid artery aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2011;42:419-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Nomura M, Mori K, Tamase A, Kamide T, Seki S, Iida Y, Nakano T, Kawabata Y, Kitabatake T, Nakajima T, Yasutake K, Egami K, Takahashi T, Takahashi M, Yanagimoto K. Pseudoaneurysm formation due to rupture of intracranial aneurysms: Case series and literature review. Neuroradiol J. 2017;30:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-e418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1907] [Cited by in F6Publishing: 3064] [Article Influence: 612.8] [Reference Citation Analysis (0)] |
| 10. | Hathidara MY, Saini V, Malik AM. Stroke in the Young: a Global Update. Curr Neurol Neurosci Rep. 2019;19:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Sun T, Chen S, Wu K, Sun M, Zhang X, You C. Trends in Incidence and Mortality of Stroke in China From 1990 to 2019. Front Neurol. 2021;12:759221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Umana GE, Cristaudo C, Scalia G, Passanisi M, Corsale G, Tomarchio L, Nicoletti G, Cicero S, Fricia M. Chronic Epidural Hematoma Caused by Traumatic Intracranial Pseudoaneurysm of the Middle Meningeal Artery: Review of the Literature with a Focus on this Unique Entity. World Neurosurg. 2020;136:198-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Aghoutane N, Lyazidi Y, Zoulati M, Bakkali T, Chtata H, Taberkant M. [Spontaneous false aneurysm of the extracranial internal carotid artery - during pregnancy]. J Med Vasc. 2019;44:233-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Sommer A, Meairs S, Gueckel F, Cornelius A, Schwartz A. Traumatic brachiocephalic pseudoaneurysm presenting with delayed stroke: case report. Neuroradiology. 2000;42:742-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Wasay M, Dai A, Dubey N, Kamran S. Acute stroke secondary to internal carotid artery pseudoaneurysm: MRI findings and treatment with endovascular coiling. J Pak Med Assoc. 2007;57:377-378. [PubMed] [Cited in This Article: ] |
| 16. | Zeleňák K, Zeleňáková J, DeRiggo J, Kurča E, Kantorová E, Poláček H. Treatment of cervical internal carotid artery spontaneous dissection with pseudoaneurysm and unilateral lower cranial nerves palsy by two silk flow diverters. Cardiovasc Intervent Radiol. 2013;36:1147-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Xue S, Shi H, Du X, Ma X. Bow Hunter's syndrome combined with ipsilateral vertebral artery dissection/pseudoaneurysm: case study and literature review. Br J Neurosurg. 2020;1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Chavan R, Ichaporia N, Vhora S, Aurangabadkar K, Kamerkar D, Hardas S, Ratta BS. Endovascular management of internal carotid artery pseudoaneurysms: Retrospective observational study. Interdiscip Neurosurg. 2021;24. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Bogaerde MV, Viaene P, Thijs V. Iatrogenic perforation of the internal carotid artery by a transarticular screw: an unusual case of repetitive ischemic stroke. Clin Neurol Neurosurg. 2007;109:466-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Helou E, Sweid A, Tjoumakaris S, Herial N, Gooch MR, Rosenwasser RH, Jabbour P. Case Report of De Novo Cavernous Carotid Artery Aneurysm After an Acute Stroke Intervention for a Carotid Occlusion. World Neurosurg. 2019;128:336-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Jeon SH, Kang HG, Kim HJ, Seo MW, Shin BS. Femoral artery pseudoaneurysm after carotid artery stenting: Two case reports. Medicine (Baltimore). 2019;98:e15309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Béres-Molnár KA, Szabó PT, Al-Muhanna N. Drug addiction. repetitive injections into carotid arteries. a rare case of stroke in young adults. Eur Stroke J. 2019;4:591. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Budinčević H, Milošević M, Pavlović T. Giant pseudoaneurysm of the external carotid artery causing stroke: A case report. J Clin Ultrasound. 2018;46:269-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Hoque R, Gonzalez-Toledo E, Minagar A, Kelley RE. Circuitous embolic hemorrhagic stroke: carotid pseudoaneurysm to fetal posterior cerebral artery conduit: a case report. J Med Case Rep. 2008;2:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Budincevic H, Piršic A, Bohm T, Trajbar T, Ivkošic A, Pavlovic T, Bielen I, Soldo-Butkovic S. Carotid Body Tumor as a Cause of Stroke. Intern Med. 2016;55:295-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Grigorian A, Kabutey NK, Schubl S, de Virgilio C, Joe V, Dolich M, Elfenbein D, Nahmias J. Blunt cerebrovascular injury incidence, stroke-rate, and mortality with the expanded Denver criteria. Surgery. 2018;164:494-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Henry JC, Franz RW. Pseudoaneurysms of the Peripheral Arteries. Int J Angiol. 2019;28:20-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Abu-Yousef MM, Wiese JA, Shamma AR. The "to-and-fro" sign: duplex Doppler evidence of femoral artery pseudoaneurysm. AJR Am J Roentgenol. 1988;150:632-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Güner A, Pala S, Gündüz S, Külahçıoğlu Ş, Güner EG. Pseudoaneurysm after carotid stenting: A case report and review of the literature. Turk Kardiyol Dern Ars. 2020;48:613-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Ogura T, Mineharu Y, Todo K, Kohara N, Sakai N. Carotid Artery Dissection Caused by an Elongated Styloid Process: Three Case Reports and Review of the Literature. NMC Case Rep J. 2015;2:21-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Rathlev NK. Penetrating neck trauma: mandatory versus selective exploration. J Emerg Med. 1990;8:75-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Song Y, Fu MY, Chen Y, Chen GP, Pan Z. [Clinical characteristics of patients with cervical vascular pseudoaneurysm after radiotherapy for nasopharyngeal carcinoma]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33:746-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Chen WL, Xu LF, Tang QL, Zhang DM. Management of carotid body tumor and pseudoaneurysm after blunt dissection. J Craniofac Surg. 2015;26:477-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 34. | DuBose J, Recinos G, Teixeira PG, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65:1561-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Kaushal S, Shake JG, Yuh DD. Mycotic innominate artery pseudoaneurysm presenting as an embolic stroke. J Thorac Cardiovasc Surg. 2005;129:945-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Rallo MS, Narayan V, Majmundar N, Al-Mufti F, Sun H, Jumah F, Raju B, Roychowdhury S, Nanda A, Gupta G. Multi-modal endovascular management of traumatic pseudoaneurysm and arteriovenous fistula of the ascending cervical artery: A technical report and review of literature. Clin Neurol Neurosurg. 2021;202:106539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Kumar A, Prabhakar A, Gupta V, Khandelwal N, Ahuja CK, Singhal M, Vyas S, Panda NK, Vaidhya PC. Endovascular management of internal carotid artery pseudoaneurysms: A single-centre experience of 20 patients. Neurol India. 2018;66:1067-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Phogat V, Gandhi A, Srivastava T, Mishva K. Endovascular management of intracranial pseudoaneurysm: an institutional experience. J Cerebrovasc Endovasc Neurosurg. 2020;22:211-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Zhong J, Islim F, Sundararajan S, Tahir N, Goddard T, Patel J. Endovascular Treatment of a Giant Extracranial Carotid Artery Pseudoaneurysm in a Child Using Vascular Plugs. Ear Nose Throat J. 2020;99:NP119-NP121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Nariai Y, Kawamura Y, Takigawa T, Hyodo A, Suzuki K. Pipeline embolization for an iatrogenic intracranial internal carotid artery pseudoaneurysm after transsphenoidal pituitary tumor surgery: Case report and review of the literature. Interv Neuroradiol. 2020;26:74-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Marnat G, Lapergue B, Sibon I, Gariel F, Bourcier R, Kyheng M, Labreuche J, Dargazanli C, Consoli A, Blanc R, Piotin M, Mazighi M, Richard S, Gory B; TITAN and ETIS Investigators*. Safety and Outcome of Carotid Dissection Stenting During the Treatment of Tandem Occlusions: A Pooled Analysis of TITAN and ETIS. Stroke. 2020;51:3713-3718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Da Ros V, Scaggiante J, Pitocchi F, Sallustio F, Lattanzi S, Umana GE, Chaurasia B, Bandettini di Poggio M, Toscano G, Rolla Bigliani C, Ruggiero M, Haznedari N, Sgreccia A, Sanfilippo G, Diomedi M, Finocchi C, Floris R. Mechanical thrombectomy in acute ischemic stroke with tandem occlusions: impact of extracranial carotid lesion etiology on endovascular management and outcome. Neurosurg Focus. 2021;51:E6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Hsiao CL, Tsai YH, Lin SK. Massive Epistaxis from Internal Carotid Pseudoaneurysm during Acute Ischemic Stroke in a Patient with Nasopharyngeal Carcinoma. Acta Neurol Taiwan. 2014;23:113-118. [PubMed] [Cited in This Article: ] |
| 44. | Ruff MW, Nasr DM, Klaas JP, Renaud DL. Internal Carotid Artery Pseudoaneurysm and Ischemic Stroke Secondary to Retropharyngeal and Parapharyngeal Abscess. J Child Neurol. 2017;32:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Kronlage C, Lorscheider J, Wagner B, Lyrer P, Peters N. Case-report: Recurrent embolic stroke from non-traumatic pseudoaneurysm of the extracranial internal carotid artery in a 60-year old woman. Eur Stroke J. 2019;4:214. [Cited in This Article: ] |
| 46. | Rigual R, Ruiz-Ares G, Rodriguez-Pardo J, Fernández-Prieto A, Navia P, Novo JR, Alonso de Leciñana M, Alonso-Singer P, Fuentes B, Díez-Tejedor E. Concurrent Cerebral, Splenic, and Renal Infarction in a Patient With COVID-19 Infection. Neurologist. 2022;27:143-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |