Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3856
Peer-review started: July 30, 2021
First decision: September 1, 2021
Revised: September 25, 2021
Accepted: March 5, 2022
Article in press: March 5, 2022
Published online: April 26, 2022
Gallbladder cancer is the most common malignant tumor in the biliary system, and it is characterized by high aggressiveness and an extremely poor prognosis. Current treatment for advanced gallbladder cancer remains unsatisfactory. Here, we report a patient with advanced gallbladder cancer who was cured by multidisciplinary treatment.
A 73-year-old male presented to our hospital with right abdominal pain for 3 d and was diagnosed with stage IVB gallbladder cancer with multiple liver metastases, peritoneum metastasis, diaphragm metastasis and lymph node metastases. The patient initially received chemotherapy, targeted therapy, 125I seed implantation and immunotherapy, as there were no specific indications for radical surgery. During these palliative therapies, the level of tumor markers gradually decreased but remained higher than the normal level, lymph node metastases gradually disappeared, and liver metastasis was gradually limited to the left liver. Finally, the patient received radical surgery with left hepatectomy, radical lymphadenectomy and partial diaphragmatic resection. To date, the patient has survived for more than six years posttreatment, the levels of tumor markers are normal, and imaging examinations show no signs of tumor recurrence.
Currently, the prognosis of advanced gallbladder cancer remains unsatisfactory. A single treatment method is not sufficient for patients with advanced gallbladder cancer. Multidisciplinary individualized treatment is essential and should be utilized for advanced gallbladder cancer patients to further improve prognosis.
Core Tip: The prognosis of advanced gallbladder cancer is extremely poor. Many clinicians and even experienced surgeons are confused and pessimistic about the treatment of advanced gallbladder cancer. Here, we report a patient with stage IVB gallbladder cancer with multiple liver metastases, peritoneum metastasis, diaphragm metastasis, and lymph node metastases who was cured by multidisciplinary treatment. Although the prognosis of metastatic gallbladder cancer remains extremely poor in the current medical field, the presented case highlights the importance of providing aggressive multidisciplinary treatment to appropriately selected patients with metastatic gallbladder cancer to achieve long-term survival.
- Citation: Zhang B, Li S, Liu ZY, Peiris KGK, Song LF, Liu MC, Luo P, Shang D, Bi W. Successful multimodality treatment of metastatic gallbladder cancer: A case report and review of literature. World J Clin Cases 2022; 10(12): 3856-3865
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3856.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3856
Gallbladder cancer is the most common malignant tumor in the biliary system, with a global average incidence of approximately 2.71/100000 and a high incidence in Chile, Japan and India[1,2]. Gallbladder cancer is characterized by its high aggressiveness and an extremely poor prognosis, and the five-year survival rates of stage Ⅰ, IVA and IVB gallbladder cancer are only 50%, 12.4% and 2.5% respectively[3-5]. Radical surgery is the only way to cure gallbladder cancer. However, gallbladder cancer has an insidious onset and is difficult to diagnose at an early stage. In fact, some patients are already in the advanced stage when the diagnosis is made[6,7]. For unresectable or metastatic gallbladder cancer, the National Comprehensive Cancer Network guidelines for hepatobiliary cancers recommend chemotherapy, radiotherapy, immunotherapy and biliary drainage as palliative therapy to prolong patient survival[8]. Here, we report a patient with stage IVB gallbladder cancer who has survived for more than six years and is currently in disease-free survival after multidisciplinary treatment.
In December 2014, a 73-year-old male presented to our hospital with right abdominal pain for 3 d.
The patient suffered right abdominal pain for 3 d.
The patient had no other significant medical history.
The patient had no family history of cancer or hepatobiliary disease.
Physical examination indicated mild tenderness in the right upper quadrant of the abdomen and positive Murphy’s sign.
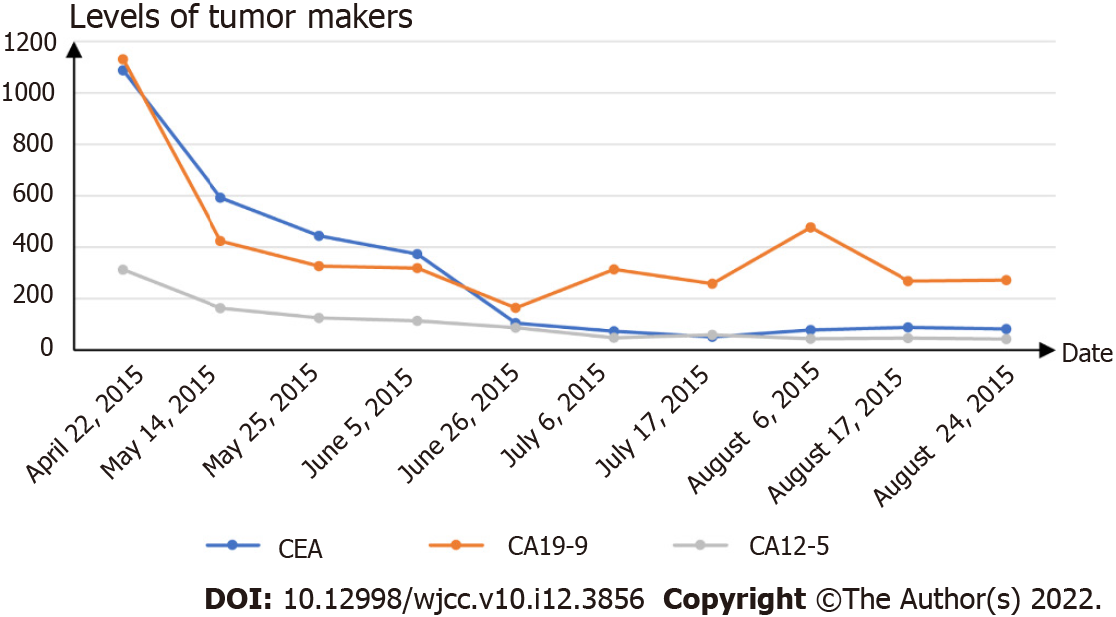
In December 2014, the laboratory examinations showed the following for tumor markers: Alpha-fetoprotein, 1.61 IU/mL (normal, 0-5.8 IU/mL); carcinoembryonic antigen (CEA), 115.8 ng/mL (normal, 0-5 ng/mL); carbohydrate antigen19-9 (CA19-9), > 1000 IU/mL (normal, 0-27 IU/mL); and CA12-5, 112.3 IU/mL (normal, 0-35 IU/mL). The blood count, liver function and kidney function examinations of the patient were at normal levels.
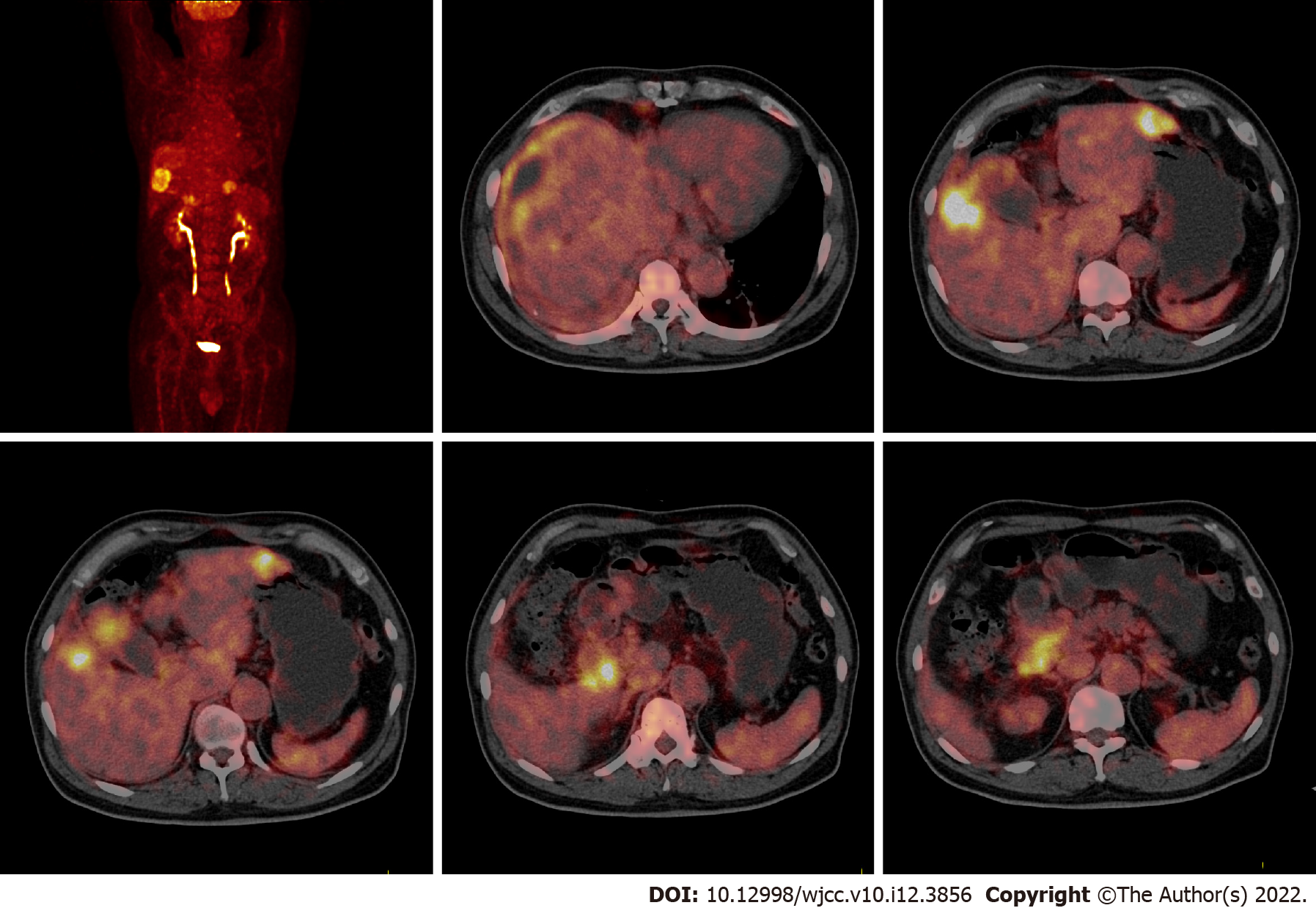
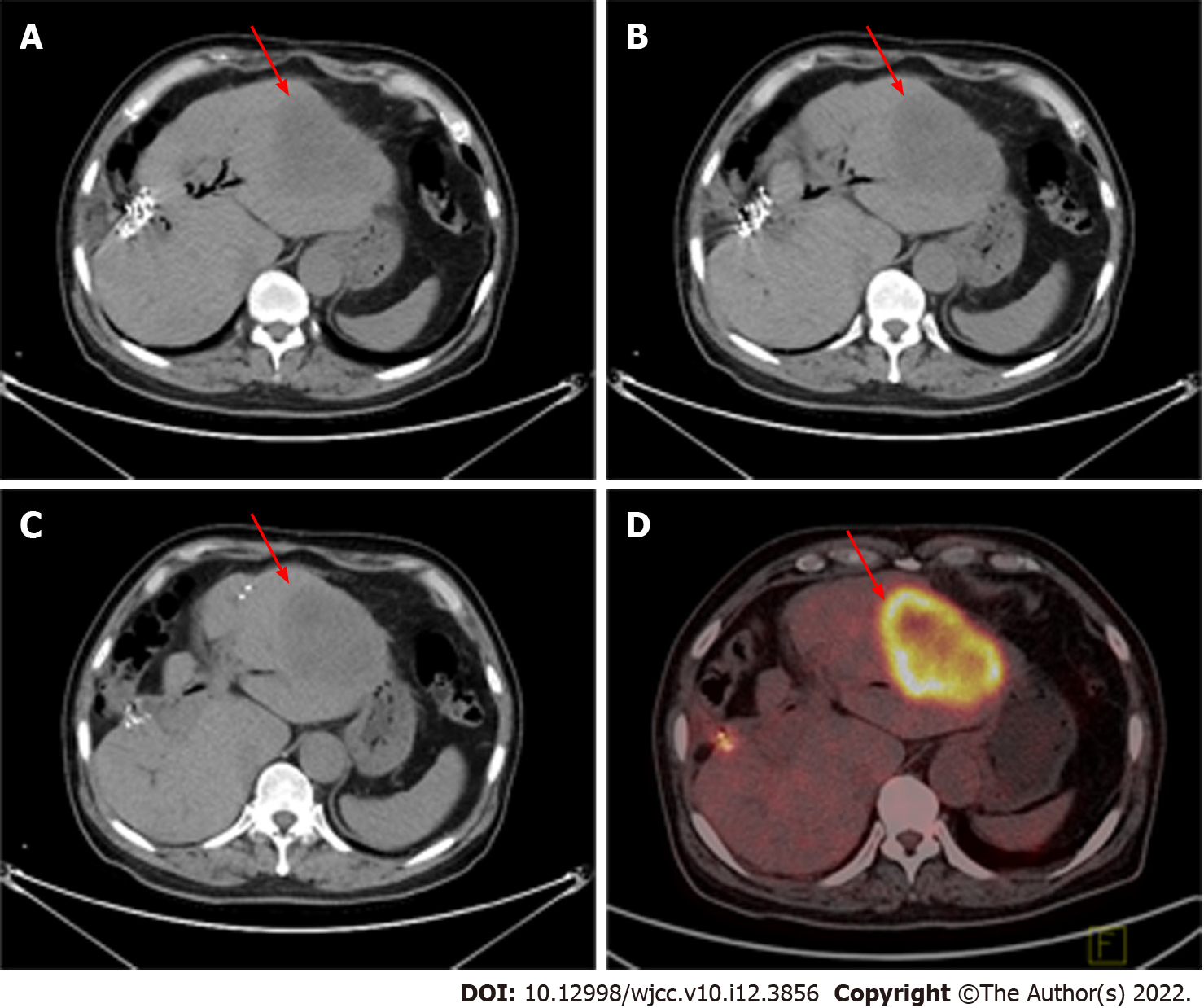
In December 2014, abdominal magnetic resonance imaging (MRI) indicated gallbladder cancer with a tumor size of approximately 3.2 cm × 4.1 cm, right liver metastasis with a tumor size of approximately 4.7 cm × 4.7 cm, left liver metastasis with a tumor size of approximately 3.6 cm × 4.2 cm, and peritoneum metastasis (Figure 1). 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) depicted gallbladder cancer (early SUVmax was 9.6, delayed SUVmax was 12.0) with multiple liver metastases (early SUVmax was 12.9, delayed SUVmax was 22.8), lymph node metastases (early SUVmax was 4.1, delayed SUVmax was 6.1), peritoneum metastasis and diaphragm metastasis (early SUVmax was 2.1, delayed SUVmax was 3.3) (Figure 2).
Based on all the above examinations and the 8th edition of the American Joint Committee on Cancer[9], the patient was diagnosed with clinical T4N2M0 and stage IVB gallbladder cancer with multiple liver metastases, peritoneum metastasis, diaphragm metastasis and lymph node metastases (Figure 3).
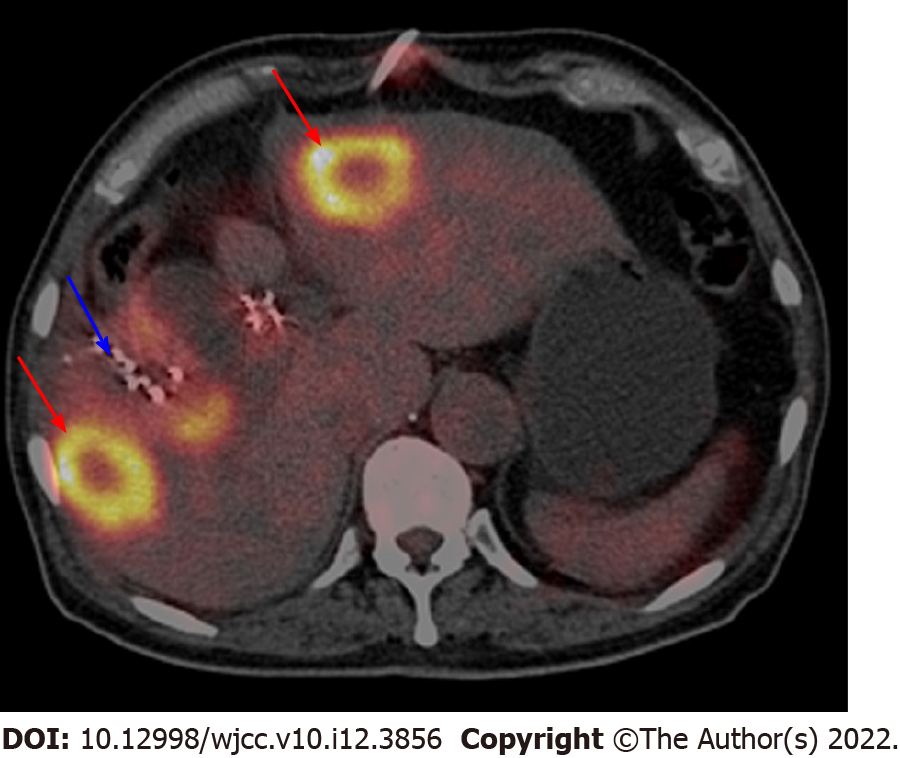
We recommended that the patient first went to the oncology department to receive palliative therapy, as there were no specific indications for radical surgery. In March 2015, the patient began receiving chemotherapy (gemcitabine 1.4 g and oxaliplatin 150 milligrams every 21 d, seven cycles) and targeted therapy (cetuximab 400 milligrams every 21 d, continuing to this day). During chemotherapy and targeted therapy, the level of tumor markers gradually decreased but remained higher than the normal level (Figure 4). In August 2015, abdominal MRI after seven cycles of chemotherapy showed that the gallbladder was malformed and that the right liver metastasis was larger than the prior scan (Figure 5). In October 2015, the patient received iodine-125 (125I) seed implantation to treat gallbladder cancer, liver metastases, and lymph node metastases. The 125I seed was implanted around the gallbladder under the guidance of CT. In January 2016, the patient began receiving immunotherapy (nivolumab 200 milligrams every 21 d, continuing to this day) and targeted therapy (apatinib 250 milligrams every day). Due to the side effects of hypertension, apatinib was in turn replaced with nintedanib and regorafenib. In March 2016, 18F-FDG-PET/CT showed that 125I seeds were around the gallbladder, but the gallbladder was not clearly visible. The left and right liver metastases still existed, and the hilar and peripancreatic lymph node metastases had disappeared (Figure 6).
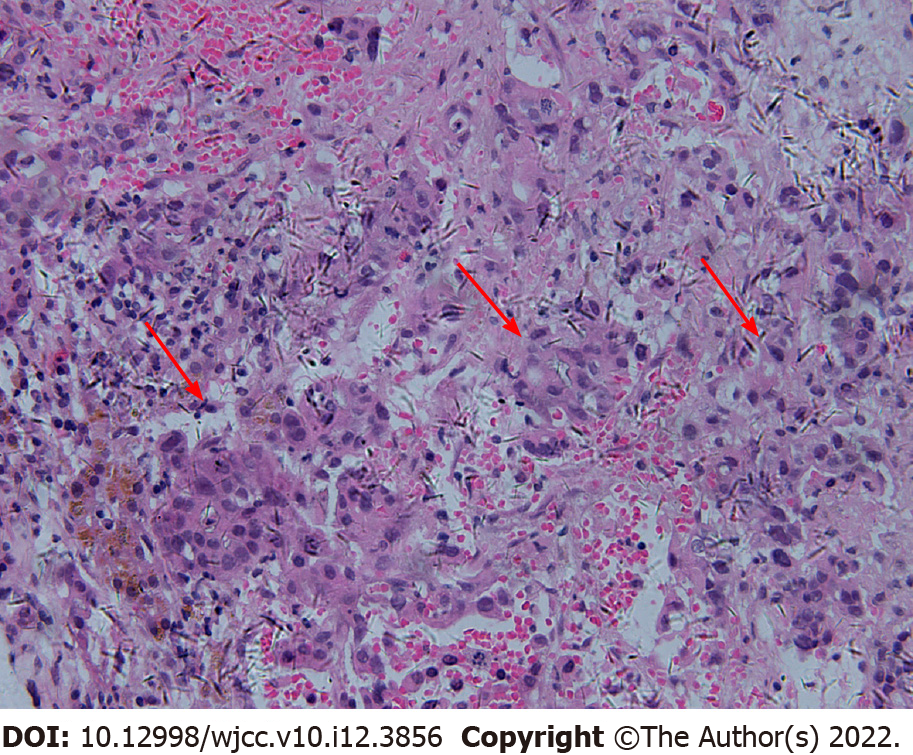
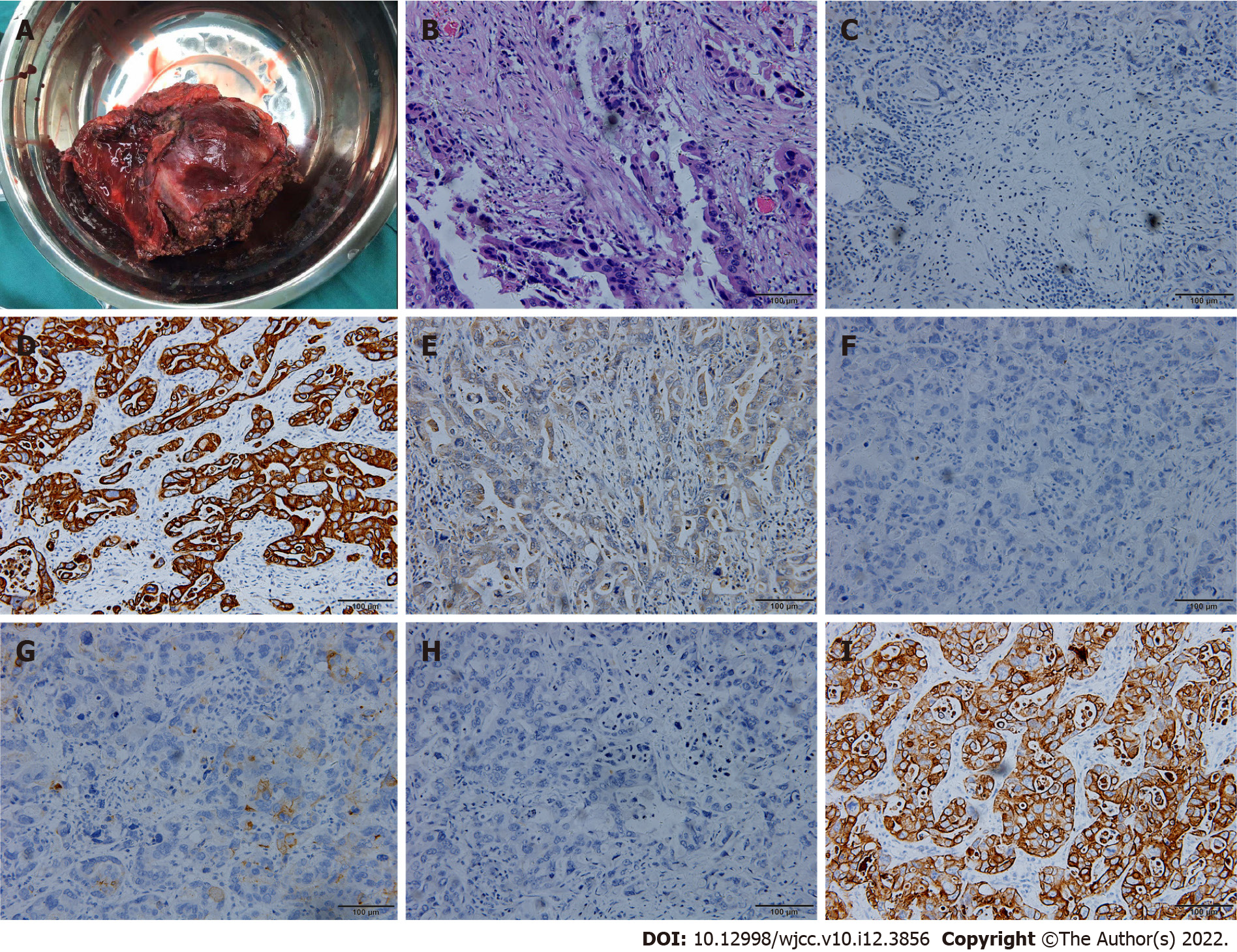
In February 2018, abdominal CT and 18F-FDG-PET/CT showed that the gallbladder had disappeared and that liver metastasis was limited to the left liver (Figure 7). We speculated that a series of adjuvant treatments led to the gradual disappearance of the gallbladder. Then the patient underwent surgery because the liver metastasis was limited to the left liver. During surgery, we detected a lesion in the left liver involving the diaphragm, and a hard mass could be palpated in the gallbladder region. Left hepatectomy with radical lymphadenectomy and partial diaphragmatic resection was subsequently conducted. The entire operation lasted approximately 3 h, and the blood loss was approximately 100 mL. The postoperative pathological examination confirmed moderate poorly differentiated cholangiocarcinoma in the left liver with invasion of the liver capsule and diaphragm, and the liver resection margin was negative (Figure 8). The postoperative immunohistochemical examination indicated ARGINASE-1 (-), CK19 (+), GPC-3 (partial +), hep-par (-), CEA (partial+), CK20 (-), and CK7 (+) (Figure 8). There were no postoperative complications, and the patient was discharged 15 d after surgery.
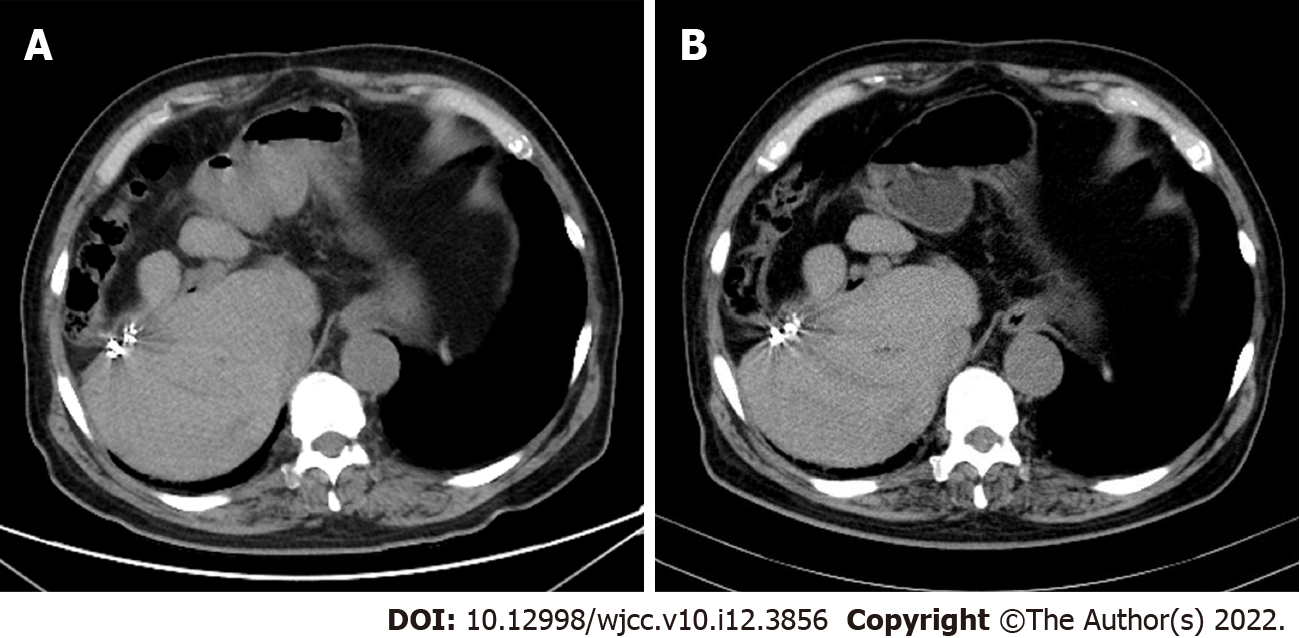
In October 2019, the patient came to our hospital for follow-up. The patient’s tumor markers had reduced to normal levels as follows: CEA, 2.23 ng/mL; CA19-9, 21.45 IU/mL; and CA12-5, 11.54 IU/mL. Additionally, abdominal CT showed no signs of tumor recurrence (Figure 9A). In March 2021, the patient’s tumor markers were still at normal levels, and abdominal CT showed no signs of tumor recurrence (Figure 9B).
The prognosis of advanced gallbladder cancer is extremely poor, and many clinicians and even experienced surgeons are uncertain and pessimistic about the treatment of advanced gallbladder cancer. A study from Kayahara et al[10] found that surgical resection did not improve the prognosis for patients with stage IV gallbladder cancer. However, some studies have shown that surgical resection can provide survival benefits for patients with advanced gallbladder cancer[11,12]. With the development of adjuvant therapies, such as chemotherapy, radiotherapy, targeted therapy and immunotherapy, a study showed that preoperative adjuvant therapy could increase the resectability and survival time of advanced malignancies[13].
Gemcitabine plus oxaliplatin or gemcitabine plus cisplatin has been shown to significantly increase the survival time of patients with advanced biliary tract cancer (BTC) and is recommended as the first-line chemotherapy for advanced BTC[14-18]. A phase III randomized controlled trial on unresectable gallbladder cancer suggested that, compared with gemcitabine plus cisplatin, gemcitabine plus oxaliplatin could provide a survival improvement, and the survival improvement median overall survival (OS) was 9 mo in the gemcitabine plus oxaliplatin group and 8.3 mo in the gemcitabine plus cisplatin group (P = 0.057)[19].
Cetuximab is a targeted therapy against epithelial growth factor receptor. A phase II study[20] involving 30 patients with unresectable advanced BTC found that cetuximab and gemcitabine plus oxaliplatin had obvious antitumor activity, and nine patients underwent potential radical secondary resection after a major response to treatment. However, a randomized, open-label, noncomparative phase II trial[21] showed that, compared to chemotherapy alone, cetuximab and gemcitabine plus oxaliplatin in patients with advanced biliary tract tumors did not show a survival improvement or a survival advantage. Whether cetuximab can benefit patients with advanced BTC is still a topic that is under research, and we anticipate that a high-quality result will benefit the future of the medical and surgical fields.
Our patient firstly received chemotherapy with gemcitabine plus oxaliplatin and targeted therapy with cetuximab because there was no specific indication for radical surgery. The tumor markers levels of the patient gradually decreased during chemotherapy and targeted therapy, which suggested that chemotherapy and targeted therapy were beneficial for the patient. After seven cycles of chemotherapy, the patient received 125I seed implantation and immunotherapy.
Radioactive seed implantation can provide continuous therapeutic doses in the tumor target area and rapidly decrease the distance of seeding. Thus, seed implantation can cause tumor cell death and delay tumor growth, and it results in only minor injuries to normal tissues[22,23]. Studies have shown that biliary stents combined with 125I seed implantation could prolong stent patency and improve survival time for patients with cholangiocarcinoma[22,24]. Furthermore, studies[25,26] have shown that compared with transcatheter arterial chemoembolization (TACE) alone, 125I seed implantation combined with TACE can better control the tumor and improve the survival time for liver cancer patients. The treatment of residual liver cancer near complex sites after TACE is challenging, but 125I seed implantation is effective and safe for patients[27].
Immunotherapy based on checkpoint blockers can block the inhibitory pathways of T-cell activation, thereby enabling tumor-reactive T cells to recognize tumor antigens and restore the antitumor immune response[28]. Immunotherapy has been indicated to benefit patients with advanced cancers such as hepatocellular carcinoma, nonsmall cell lung cancer and urothelial carcinoma, but the efficacy of immunotherapy for advanced BTC is still in the exploratory stage[29]. A nonrandomized, multicenter, open-label, phase Ⅰ study[30] showed that, compared with nivolumab only, nivolumab and cisplatin plus gemcitabine could significantly increase OS from 5.2 mo to 15.4 mo and increase PFS from 1.4 mo to 4.2 mo for unresectable or recurrent BTC.
Our patient eventually underwent radical surgery after a series of palliative treatments. Chemotherapy, targeted therapy, 125I seed implantation and immunotherapy certainly played an important role in facilitating radical surgery in this patient. However, the patient underwent a very long and complicated treatment process. It is difficult to identify the specific role of each treatment. More studies are needed to investigate this issue, and we look forward to future studies on multidisciplinary treatment for advanced gallbladder cancer.
We reported a patient with advanced gallbladder cancer cured by multidisciplinary treatment, which was extremely rare and inspiring. Although the prognosis of metastatic gallbladder cancer remains extremely poor in the current medical field, the presented case highlights the importance of providing aggressive multidisciplinary treatment to appropriately selected patients with metastatic gallbladder cancer to achieve long-term survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chrcanovic BR, Sweden S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Weaver AJ, Stafford R, Hale J, Denning D, Sanabria JR; GBD Collaborators. Geographical and Temporal Variation in the Incidence and Mortality of Hepato-Pancreato-Biliary Primary Malignancies:1990-2017. J Surg Res. 2020;245:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Zaidi MY, Maithel SK. Updates on Gallbladder Cancer Management. Curr Oncol Rep. 2018;20:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Imaoka Y, Kuranishi F, Miyazaki T, Yasuda H, Ohno T. Long-lasting complete response status of advanced stage IV gall bladder cancer and colon cancer after combined treatment including autologous formalin-fixed tumor vaccine: two case reports. World J Surg Oncol. 2017;15:170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Hickman L, Contreras C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg Clin North Am. 2019;99:337-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Kawamoto M, Wada Y, Koya N, Takami Y, Saitsu H, Ishizaki N, Tabata M, Onishi H, Nakamura M, Morisaki T. Long-term survival of a patient with recurrent gallbladder carcinoma, treated with chemotherapy, immunotherapy, and surgery: a case report. Surg Case Rep. 2018;4:115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Zhong Y, Wu X, Li Q, Ge X, Wang F, Wu P, Deng X, Miao L. Long noncoding RNAs as potential biomarkers and therapeutic targets in gallbladder cancer: a systematic review and meta-analysis. Cancer Cell Int. 2019;19:169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Erdem S, White RR. Incidental Gallbladder Cancer: Permission to Operate. Ann Surg Oncol. 2020;27:980-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 395] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 9. | Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2018: 308. [Cited in This Article: ] |
| 10. | Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer. 2007;110:572-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Shimizu H, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, Mitsuhashi N, Takeuchi D, Suda K, Yoshioka I, Miyazaki M. Aggressive surgical approach for stage IV gallbladder carcinoma based on Japanese Society of Biliary Surgery classification. J Hepatobiliary Pancreat Surg. 2007;14:358-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Mao W, Deng F, Wang D, Gao L, Shi X. Treatment of advanced gallbladder cancer: A SEER-based study. Cancer Med. 2020;9:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - A systematic review. Eur J Surg Oncol. 2019;45:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q, Zhu F, Li X, Qian X, Hu J, Zhao F, Mao W, Sun J, Wang J, Han G, Li C, Xia Y, Seesaha PK, Zhu D, Li H, Zhang J, Wang G, Wang X, Shu Y. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P, Chaudhary SP. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581-4586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 16. | Weigt J, Malfertheiner P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2010;4:395-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2617] [Cited by in F6Publishing: 2756] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 18. | Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, Bridgewater J, Okusaka T. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 19. | Sharma A, Kalyan Mohanti B, Pal Chaudhary S, Sreenivas V, Kumar Sahoo R, Kumar Shukla N, Thulkar S, Pal S, Deo SV, Pathy S, Ranjan Dash N, Kumar S, Bhatnagar S, Kumar R, Mishra S, Sahni P, Iyer VK, Raina V. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: Results of a phase III randomised controlled trial. Eur J Cancer. 2019;123:162-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K, Roka R, Freimann-Pircher S, Gruenberger T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11:1142-1148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, Fartoux L, Faivre S, Blanc JF, Viret F, Assenat E, Seufferlein T, Herrmann T, Grenier J, Hammel P, Dollinger M, André T, Hahn P, Heinemann V, Rousseau V, Ducreux M, Pignon JP, Wendum D, Rosmorduc O, Greten TF; BINGO investigators. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 22. | Zhang W, Yang ZQ, Shi HB, Liu S, Zhou WZ, Zhao LB. Placement of ¹²⁵I seed strands and stents for a type IV Klatskin tumor. World J Gastroenterol. 2015;21:373-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Han T, Yang X, Xu Y, Zheng Z, Yan Y, Wang N. Therapeutic value of 3-D printing template-assisted 125I-seed implantation in the treatment of malignant liver tumors. Onco Targets Ther. 2017;10:3277-3283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Wang HW, Li XJ, Li SJ, Lu JR, He DF. Biliary stent combined with iodine-125 seed strand implantation in malignant obstructive jaundice. World J Clin Cases. 2021;9:801-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Chen L, Sun T, Kan X, Chen S, Ren Y, Cao Y, Yan L, Liang B, Xiong B, Zheng C. Transarterial chemoembolization combined with iodine-125 seed implantation for patients with hepatocellular carcinoma: a retrospective controlled study. J Int Med Res. 2020;48:300060520944309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Liu W, Xiu N, Zhao J, Zhao L. Enhanced therapeutic effect of transcatheter arterial chemoembolization combined with radioactive I-125 seed implantation on liver cancer. Oncol Lett. 2020;20:2493-2498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Ren Y, Dong X, Chen L, Sun T, Alwalid O, Kan X, Su Y, Xiong B, Liang H, Zheng C, Han P. Combined Ultrasound and CT-Guided Iodine-125 Seeds Implantation for Treatment of Residual Hepatocellular Carcinoma Located at Complex Sites After Transcatheter Arterial Chemoembolization. Front Oncol. 2021;11:582544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Lee HT, Lee SH, Heo YS. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules. 2019;24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 29. | Rizzo A, Ricci AD, Brandi G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:527-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, Okano N, Kimura K, Asada S, Namba Y, Okusaka T, Furuse J. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:611-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |