Published online Mar 26, 2016. doi: 10.5662/wjm.v6.i1.112
Peer-review started: December 21, 2015
First decision: January 21, 2016
Revised: February 1, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: March 26, 2016
Processing time: 90 Days and 18.9 Hours
Natural peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists are found in food and may be important for health through their anti-inflammatory properties. Curcumin (Cur) is a bright yellow spice, derived from the rhizome of Curcuma longa Linn. It has been shown to have many biological properties that appear to operate through diverse mechanisms. Some of these potentially beneficial effects of Cur are due to activation of the nuclear transcription factor PPAR-γ. It is reported (using in vitro and in vivo models) that Cur plays a potential role against several diseases. In this review article, we present the current literature on the effects of Cur on the modulation of inflammatory processes that are mediated through PPAR-γ.
Core tip: In this short review, we highlight the potential antioxidant and anti-inflammatory properties of curcumin (Cur), discussing its impact on peroxisome proliferator-activated receptor-γ (PPAR-γ) receptor function and its effects in vitro and in vivo. Cur affects the PPAR-γ gene and prevents cell growth through effects on the cell cycle and induction of apoptosis. It is also well-established that Cur has anti-inflammatory effects in vivo through regulation of the PPAR-γ receptor, which leads to the suppression of nuclear factor kappa B, a pro-inflammatory mediator.
- Citation: Mazidi M, Karimi E, Meydani M, Ghayour-Mobarhan M, Ferns GA. Potential effects of curcumin on peroxisome proliferator-activated receptor-γ in vitro and in vivo. World J Methodol 2016; 6(1): 112-117
- URL: https://www.wjgnet.com/2222-0682/full/v6/i1/112.htm
- DOI: https://dx.doi.org/10.5662/wjm.v6.i1.112
Curcumin (diferuloylmethane) (Cur) is an orange pigment extractable from turmeric. Curcuma is derived from the word “Kourkoum”. Due to its color, curcuma is sometimes referred to in Europe as “Indian Saffron”. As a result of its chemical and biological properties, Cur is known to contain several potential important phytochemical compounds[1-5]. Cur is a lipophilic polyphenol, is poorly soluble in water and stable at an acidic pH[6]. A critical review of Cur suggests that the compound has potential as a modulator of the activity of many vital bio-macromolecular targets involved in homeostasis of mammalian physiology[7]. Dietary polyphenols have recently received more attention because of their potentially protective characteristics against metabolic diseases[8].
Cur has been reported to be safe at dosages of up to 8 g/d in human studies and there is no evidence of resistance. Nevertheless, bioavailability is a major concern as 75% of Cur is excreted in the stool[9,10]. Besides its dietary use, Cur has been considered to have beneficial properties, including anti-inflammatory, antioxidant, antineoplastic, pro and anti-apoptotic, anti-angiogenic, cytotoxic, immune-modulatory and antimicrobial effects, through the modulation of various kinds of targets, including growth factors, enzymes and genes such as STAT3, peroxisome proliferator-activated receptor-γ (PPAR-γ) and nuclear factor kappa B (NF-κB)[11,12]. It also has a strong anti-inflammatory effect that inhibits several mediators of the inflammatory response[13-15]. Due to its low solubility in water and therefore poor oral bioavailability, nanoparticles and liposomes have been suggested as potential ways of improving its efficacy[16].
PPARs are a class of proteins that are usually activated by their respective ligands and function within the cell nuclei for controlling metabolism, development and homeostasis. PPARs heterodimerize with the retinoid X receptor and bind to PPAR responsive element in the regulatory region of target genes that function in different natural courses, such as adipogenesis, immune response and both cell growth and differentiation[17,18]. There are 3 major isoforms of PPARs in mammals, namely PPARα, PPAR-γ and PPARα/γ. PPAR-α can improve triglyceride concentration and also has some roles in energy homeostasis, whereas activation of PPAR-α/γ improves fatty acid hemostasis[19]. PPAR-γ is involved in lipid anabolism, adipocyte differentiation inflammation and immune response[20]. PPAR-α is triggered by a wide diversity of fatty acids or their metabolites and governs metabolic processes implicated in glucose and lipid metabolism and adipose mass control by modulating the expression of a huge quantity of target genes. Furthermore, PPAR-γ is a molecular target for anti-diabetic thiazolidinedione molecules that selectively bind this nuclear receptor to improve systemic insulin sensitivity and glucose tolerance. Accordingly, the specific position of PPAR-γ in systemic metabolic control is due to its pivotal role in the homeostasis control of glucose and lipid homeostasis, lipid storage and adipogenesis[21]. Lately, PPAR-γ has been recognized to be the major player with a key role in the immune response because of its capability to prevent the production of inflammatory substances[22].
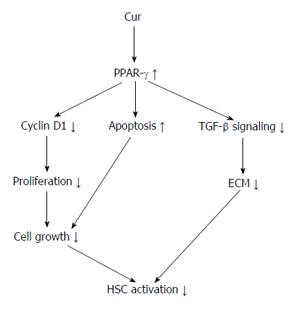
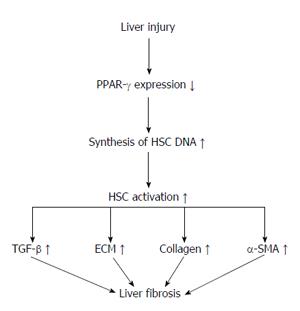
Hepatic stellate cells (HSCs) are located near to hepatic epithelial cells. In a normal liver, HSCs contain many vitamin A lipid droplets. When the liver is injured, HSCs receive signals from damaged cells in the liver to change into activated myofibroblast-like cells[23,24]. In addition, HSCs secrete growth factors and help in the maintenance of liver cells. In liver disease, extended and frequent activation of HSCs causes liver fibrosis that may eventually result in organ failure and death[25,26]. Activation of hepatic HSCs is a key step in liver collagen production and fibrosis formation[27-31]. Hepatic fibrosis is also a necessary step in the development of hepatic cirrhosis. Thus, treatment of chronic liver diseases depends on the prevention and treatment of fibrosis[32]. Some studies showed that HSC activation significantly reduces the expression of PPAR-γ and that PPAR-γ agonists inhibit HSC activation, resulting in reduced expression of α-SMA and collagen, as well as reduced cell propagation and development of hepatic fibrosis. In normal liver tissues, PPAR-γ is expressed highly in quiescent HSCs. Moreover, increased PPAR-γ expression reduces the synthesis of HSC DNA and results in the diminished expression of collagen and the transforming growth factor (TGF)-1β. At the same time, PPAR-γ is also involved in the apoptosis of HSCs through a variety of mechanisms[33-36]. Some experiments have confirmed that Cur may prevent the proliferation of HSCs whilst also increasing their apoptosis[37]. A further study has shown that Cur increases the expression of PPAR-γ and revives the trans-activating activity in activated HSC, which is essential for the anti-inflammatory and antioxidant effects on reserve for HSC propagation and growth[38] (Figure 1).
In this review article, we present the current literature to display the role of Cur on modulation of inflammatory processes that are mediated through PPAR-γ.
HSCs are activated when gene expression and phenotype changes render the quiescent cells responsive to other cytokines. Kupffer cells provide the potential source of paracrine stimuli for HSCs because they express TGF-β[24,25,39-41]. During HSC activation, regulatory pathways including epigenetic regulation of (NF-κB) and reduction in PPAR-γ expression modulate the expression of many genes, including TGF-1β and MMP-2[42-46].
Many in vitro studies have shown that Cur inhibits cell proliferation and induces apoptosis of stimulated HSC. However, the mechanism and action of Cur on HSC growth in vitro is not well defined. Numerous mechanisms have been recognized for the inhibition of TGF-1β signaling via Cur, including PPAR-γ activation. Cur inhibits NF-κB, leptin and insulin and mediates HSC activation by stimulating PPAR-γ activity[38,47-51] (Figure 2).
Zheng et al[52] confirmed that inhibiting PPAR-γ stimulation abrogated the effects of Cur on the stimulation of apoptosis and prevention of the expression of ECM genes in activated HSC in vitro. They also showed that Cur repressed the gene expression of TGF-β receptors and disturbed the TGF-β signaling pathway in stimulated HSC, which is facilitated by PPAR-γ stimulation[52]. Zhang et al[37] established that Cur improved fibrotic injury and sinusoidal angiogenesis in the rodent liver when fibrosis was initiated by carbon tetrachloride. Cur decreased the expression of a number of angiogenic factors in the fibrotic liver. Moreover, in vitro investigation showed that the sustainability and vascularization of rodent liver sinusoidal endothelial cells and angiogenesis in rodents were not diminished by Cur. These findings demonstrated that HSCs could be a possible target for Cur. Moreover, other studies have shown that Cur can inhibit vascular endothelial growth factor expression in HSCs associated with interrupting the mammalian target of rapamycin pathway. PPAR-γ activation was reported to be essential for Cur to prevent the angiogenesis in HSCs. The authors determined that Cur reduced sinusoidal angiogenesis in liver fibrosis probably by HSCs via a PPAR-γ activation-dependent pathway. Also, other studies showed that PPAR-γ could be a target molecule for decreasing pathological angiogenesis in liver fibrosis for rodents[37]. These studies offer new perspectives into the mechanisms that underpin prevention of HSC activation by Cur and PPAR-γ ligands and inhibit HSC activation and liver fibrosis. To convert stimulated HSCs to a quiescent state or to induce apoptosis may be a dangerous approach for anti-fibrotic treatment.
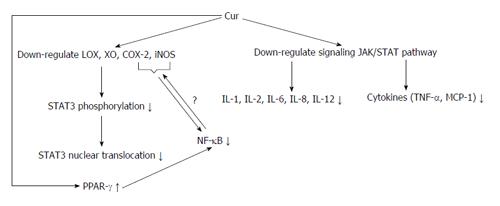
It appears that the hydroxyl and methoxy residues of Cur are accountable for its antioxidant and anti-inflammatory effects[53,54]. Some of the effects of Cur are through the JAK/STAT pathway, which can decrease pro-inflammatory interleukins and cytokines. Moreover, Cur suppresses the inflammatory response by decreasing the activity of cyclooxygenase-2 (COX-2) and lipoxygenase, resulting in inhibition of STAT3 phosphorylation and consequent STAT3 nuclear translocation[55-58]. Cur suppression of COX-2 and inducible nitric oxide synthase may be via the inhibition of the NF-κB activation by this polyphenol group.
Kawamori et al[59] have shown that dietary Cur inhibits phospholipase A2 and affects COX and lipoxygenase actions. Cur decreases COX-2 expression at the transcriptional level[13]. Cur is supposed to inhibit NF-κB and pro-inflammatory substances by hindering phosphorylation of inhibitory factor I kappa B kinase. The growing incidence of allergic disease, combined with promising outcomes from RCTs, proposes that natural PPAR-γ agonists found in the diet might be helpful by acting as anti-inflammatory factors[59-61].
Cur has been reported to trigger PPAR-γ but whether or not it is a ligand for it is still debated and further experimental work is required in this regard (Figure 3). Moreover, the exact mechanisms by which Cur stimulates PPAR-γ expression are still unknown. Given the important role of Cur, there may be two ways. Cur binds to its own receptor and the complex stimulates the up-regulation of PPAR-γ, or Cur is a ligand of PPAR-γ leading to the stimulation of PPAR-γ[62,63]. A summary of the possible molecular targeting of Cur and PPAR-γ modulated by Cur is shown in Table 1. Investigators have described the in vitro anti-inflammatory pathways of Cur and they suggest that it was reached mostly through the down-regulation of NF-κB[4,16]. Most experiments have shown that the anti-inflammatory effect of Cur is attributed to PPAR-γ activation[64]. Recent experimental data have shown that Cur has an antitumor effect in pancreatic cancer by inhibiting propagation and down-regulating NF-κB and its products[65]. Nevertheless, it is reasonable to suggest that Cur prompted an anti-inflammatory effect through the up-regulation of PPAR-γ which is closely related to the NF-κB pathway.
| Transcription factors | Growth factor/or cytokines | Proteins/or protein kinase pathway | Inflammatory mediators | Enzymes |
| STAT3 ↓ | TGF-β↓ | Cyclin D1 ↓ | IL-1 ↓ | LOX ↓ |
| NF-κB ↓ | TNF-α↓ | Collagen ↓ | IL-2 ↓ | XO ↓ |
| MCP-1 ↓ | LDL ↓ | IL-6 ↓ | COX-2 ↓ | |
| Insulin ↓ | IL-8 ↓ | iNOS ↓ | ||
| Leptin ↓ | LOX ↓ | |||
| JAK/STAT ↓ |
In this short review, we have highlighted the potential antioxidant and anti-inflammatory activities of Cur and discussed Cur’s significant impact on PPAR-γ receptor function. Cur prompts the expression of the PPAR-γ gene, causing its activation in cells to activate HSCs and hepatic fibrosis. This combined action of Cur and PPAR-γ prevents cell growth from the stimulation of the cell cycle and induction of apoptosis. It is also well-established that Cur has anti-inflammatory effects in vivo through regulation of the PPAR-γ receptor, which leads to the suppression of NF-κB, a pro-inflammatory mediator.
P- Reviewer: Chan WH, Chintana PY S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol. 2000;71:23-43. [PubMed] |
| 2. | Himesh S, Sharan PS, Mishra K, Govind K, Singhai AK. Qualitative and quantitative profil curcumin from ethanolic extract of curcuma longa. Int Res J Pharm Chem. 2011;2:180. |
| 3. | Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 884] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 4. | Shishodia S. Molecular mechanisms of curcumin action: gene expression. Biofactors. 2013;39:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Talero E, Ávila-Roman J, Motilva V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Curr Pharm Des. 2012;18:3939-3965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141-153. [PubMed] |
| 7. | Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 749] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 8. | Sohrab G, Hosseinpour-Niazi S, Hejazi J, Yuzbashian E, Mirmiran P, Azizi F. Dietary polyphenols and metabolic syndrome among Iranian adults. Int J Food Sci Nutr. 2013;64:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009;26:2438-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Vyas A, Dandawate P, Padhye S, Ahmad A, Sarkar F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr Pharm Des. 2013;19:2047-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 799] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 13. | Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1484] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 14. | Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6:93-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 15. | Chuang SE, Cheng AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem Toxicol. 2000;38:991-995. [PubMed] |
| 16. | Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Med. 2014;80:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Wu J, Chen L, Zhang D, Huo M, Zhang X, Pu D, Guan Y. Peroxisome proliferator-activated receptors and renal diseases. Front Biosci (Landmark Ed). 2009;14:995-1009. [PubMed] |
| 18. | Yang J, Chen L, Zhang X, Zhou Y, Zhang D, Huo M, Guan Y. PPARs and Female Reproduction: Evidence from Genetically Manipulated Mice. PPAR Res. 2008;2008:723243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 706] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 20. | Park JI. The role of 15d-PGJ2, a natural ligand for peroxisome proliferator-activated receptor γ (PPARγ), in cancer, in Cellular and Genetic Practices for Translational Medicine. 2011;169-195. |
| 21. | El Akoum S. PPAR Gamma at the Crossroads of Health and Disease: A Masterchef in Metabolic Homeostasis. Endocrinol Metab Synd. 2014;3:126. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Majdalawieh A, Ro HS. PPARgamma1 and LXRalpha face a new regulator of macrophage cholesterol homeostasis and inflammatory responsiveness, AEBP1. Nucl Recept Signal. 2010;8:e004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 24. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2196] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 25. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 737] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 26. | Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2470] [Cited by in RCA: 2626] [Article Influence: 202.0] [Reference Citation Analysis (0)] |
| 27. | Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56:1171-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448-9453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 634] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 29. | Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, Pradere JP, Friedman RA, Schwabe RF. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073-1083.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 30. | Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1625] [Cited by in RCA: 1527] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 31. | Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 570] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 32. | Wang Z, Xu JP, Zheng YC, Chen W, Sun YW, Wu ZY, Luo M. Peroxisome proliferator-activated receptor gamma inhibits hepatic fibrosis in rats. Hepatobiliary Pancreat Dis Int. 2011;10:64-71. [PubMed] |
| 33. | Chen H, He YW, Liu WQ, Zhang JH. Rosiglitazone prevents murine hepatic fibrosis induced by Schistosoma japonicum. World J Gastroenterol. 2008;14:2905-2911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Nan YM, Fu N, Wu WJ, Liang BL, Wang RQ, Zhao SX, Zhao JM, Yu J. Rosiglitazone prevents nutritional fibrosis and steatohepatitis in mice. Scand J Gastroenterol. 2009;44:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Sun K, Wang Q, Huang XH. PPAR gamma inhibits growth of rat hepatic stellate cells and TGF beta-induced connective tissue growth factor expression. Acta Pharmacol Sin. 2006;27:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Wang X, Huang G, Mei S, Qian J, Ji J, Zhang J. Over-expression of C/EBP-alpha induces apoptosis in cultured rat hepatic stellate cells depending on p53 and peroxisome proliferator-activated receptor-gamma. Biochem Biophys Res Commun. 2009;380:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Zhang F, Zhang Z, Chen L, Kong D, Zhang X, Lu C, Lu Y, Zheng S. Curcumin attenuates angiogenesis in liver fibrosis and inhibits angiogenic properties of hepatic stellate cells. J Cell Mol Med. 2014;18:1392-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20-G30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2160] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 40. | Rockey DC. Translating an understanding of the pathogenesis of hepatic fibrosis to novel therapies. Clin Gastroenterol Hepatol. 2013;11:224-31.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Safadi R, Friedman SL. Hepatic fibrosis--role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 43. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1292] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 44. | Attia YM, Elalkamy EF, Hammam OA, Mahmoud SS, El-Khatib AS. Telmisartan, an AT1 receptor blocker and a PPAR gamma activator, alleviates liver fibrosis induced experimentally by Schistosoma mansoni infection. Parasit Vectors. 2013;6:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Baroni GS, D’Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189-1199. [PubMed] |
| 46. | Calleja MA, Vieites JM, Montero-Meléndez T, Torres MI, Faus MJ, Gil A, Suárez A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br J Nutr. 2013;109:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 47. | Lin J, Tang Y, Kang Q, Feng Y, Chen A. Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. Br J Pharmacol. 2012;166:2212-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 49. | Tang Y, Zheng S, Chen A. Curcumin eliminates leptin’s effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology. 2009;150:3011-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Kang Q, Chen A. Curcumin suppresses expression of low-density lipoprotein (LDL) receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cells. Br J Pharmacol. 2009;157:1354-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Lin J, Zheng S, Chen A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress. Lab Invest. 2009;89:1397-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Zheng S, Chen A. Activation of PPARgamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J. 2004;384:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Rahman I, Biswas SK. Regulation of Inflammation, Redox, and Glu-cocorticoid Signaling by Dietary Polyphenols. Boca Raton: CRC Press 2009; . [DOI] [Full Text] |
| 54. | Grynkiewicz G, Ślifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim Pol. 2012;59:201-212. [PubMed] |
| 55. | Maradana MR, Thomas R, O’Sullivan BJ. Targeted delivery of curcumin for treating type 2 diabetes. Mol Nutr Food Res. 2013;57:1550-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Sahebkar A. Why it is necessary to translate curcumin into clinical practice for the prevention and treatment of metabolic syndrome? Biofactors. 2013;39:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Soetikno V, Suzuki K, Veeraveedu PT, Arumugam S, Lakshmanan AP, Sone H, Watanabe K. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today. 2013;18:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Ghorbani Z, Hekmatdoost A, Mirmiran P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int J Endocrinol Metab. 2014;12:e18081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-412. [PubMed] |
| 60. | Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82-86. [PubMed] |
| 61. | Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat Res. 2009;669:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288:G447-G456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Narala VR, Smith MR, Adapala RK, Ranga R, Panati K, Moore BB, Leff T, Reddy VD, Kondapi AK, Reddy RC. Curcumin is not a ligand for peroxisome proliferator-activated receptor-gamma. Gene Ther Mol Biol. 2009;13:20-25. |
| 64. | Jacob A, Wu R, Zhou M, Wang P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-gamma Activation. PPAR Res. 2007;2007:89369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 65. | Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |