Published online Jun 26, 2015. doi: 10.5662/wjm.v5.i2.101
Peer-review started: March 5, 2015
First decision: April 10, 2015
Revised: April 28, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: June 26, 2015
Helicobacter pylori is one of the most commonly seen bacterium worldwide. It’s in the etiology of multiple gastrointestinal diseases, ranging from gastritis to gastric carcinoma. The antimicrobial therapies, which are frequently prescribed empirically, are losing their effectivity as a result of the increasing antimicrobial resistance. As the standard triple therapy is now left especially in areas with high-clarithromycin resistance due to decreased eradication rates, quadruple therapies are recommended in most regions of the world. Alternatively, concomitant, sequential and hybrid therapies are used. There is still a debate going on about the use of levofloxacin-based therapy in order to prevent the increase in quinolone resistance. If no regimen can achieve the desired eradication rate, culture-guided individualized therapies are highly recommended. Probiotics, statins and n-acetylcysteine are helpful as adjuvant therapies in order to increase the effectiveness of the eradication therapy. Herein, we focused on different eradication regimens in order to highlight the current Helicobacter pylori treatment.
Core tip: In this review, we focused on different treatment regimens used for Helicobacter pylori eradication. The worldwide increase in antibiotic resistance, especially clarithromycin, caused change in the preferred initial treatments. The efficiency of bismuth-quadruple therapy, sequential, concomitant and hybrid therapies are emphasized in relation to each other. In addition, adjuvant therapies to increase the efficiency are reviewed. In conclusion, the optimal approach for eradication was found to be the individualized therapy.
-
Citation: Ermis F, Tasci ES. Current
Helicobacter pylori treatment in 2014. World J Methodol 2015; 5(2): 101-107 - URL: https://www.wjgnet.com/2222-0682/full/v5/i2/101.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i2.101
Helicobacter pylori (H. pylori), a gram-negative microaerophilic spiral bacillus discovered in 1983, affects nearly 50% of the world’s population. While the prevalence is as high as 90% in developing countries, it is below 40% in developed countries, besides Japan[1]. Many studies have revealed a strong relation between the organism infection and gastric disorders, especially functional dyspepsia, peptic ulcer disease, gastric carcinoma and mucosa associated lymphoid tissue-lymphoma[2]. Moreover, extra-digestive diseases are also associated with H. pylori; idiopathic thrombocytopenic purpura and idiopathic iron deficiency anemia[3]. Therefore, eradication of H. pylori is an important issue, which still remains unsolved. Today, there is still not a single optimal antibiotic treatment for eradication. Herein, we focused on many articles published over the past years on H. pylori eradication regimens and their efficacy.
In the 90’s, Bazzoli et al[4] first proposed the clarithromycin based standard triple therapy -clarithromycin, proton pump inhibitor (PPI) plus amoxicillin or metronidazole given for 7-14 d - which then became the gold standard in the treatment of H. pylori (Table 1). While the high eradication success (> 80%), optimal safety profile and relative simplicity made this regimen one of the standard of care treatments for first-line eradication of H. pylori, the raise in clarithromycin resistance in the 2000’s caused a significant decline in the efficacy of this standard regimen[5]. In their study, Lee et al[6] reported the factors causing treatment failure as; age ≥ 50 years, female gender, body mass index < 25 kg/m2, amoxicillin, and/or clarithromycin resistance by univariate analysis. On the other hand, clarithromycin resistance was the only worthy parameter found by multivariate analysis. Clarithromycin works by interrupting the bacterial protein synthesis and resistance is caused by a mutation in the organism, which prevents the binding of the antibiotic to the ribosome of H. pylori[7]. The use of clarithromycin for respiratory and gastrointestinal infections causes the increased resistance rates[8]. High bacterial load, strain types, high gastric acidity and low compliance are the other contributors to eradication failure[9]. New evidence suggests that treatment failure may be due to the ability of the bacterium to control T-cell responses[10]. The clarithromycin resistance rate is variable in different parts of the world; the most recent report from European Helicobacter Study Group stated the primary resistance rate for clarithromycin as 17.5%[11]. The threshold of 15%-20% prevalence is used to classify low or high clarithromycin resistance[12]. That determines the approach to H. pylori eradication.
| Standard triple therapy (7-14 d) | PPI - standard dose, bid Clarithromycin - 500 mg, bid Amoxicillin - 1 g, bid |
| Bismuth quadruple Therapy (10-14 d) | PPI - standard dose, bid Bismuth - standard dose, qid Tetracycline - 500 mg, qid Metronidazole - 500 mg, tid |
| Sequential therapy (5-d dual therapy followed by a 5-d triple therapy) | Dual therapy; PPI - standard dose, bid Amoxicillin - 1 g, bid Triple therapy; PPI - standard dose, bid Clarithromycin - 500 mg, bid Metronidazole - 500 mg, bid |
| Concomitant therapy (7-10 d) | PPI - standard dose, bid Clarithromycin - 500 mg, bid Amoxicillin - 1 g, bid Metronidazole - 500 mg, bid |
| Hybrid therapy (7-d dual therapy followed by a 7-d quadruple therapy) | Dual therapy; PPI - standard dose, bid |
| Amoxicillin - 1 g, bid | |
| Triple therapy; PPI - standard dose, bid | |
| Amoxicillin - 1 g, bid | |
| Clarithromycin - 500 mg, bid | |
| Metronidazole - 500 mg, bid | |
| Levofloxacin-based triple therapy (10-d) | PPI - standard dose, bid |
| Levofloxacin - 500 mg, qd | |
| Amoxicillin - 1 g, bid | |
| Rifabutin-based triple therapy (7-14 d) | PPI - standard dose, bid |
| Amoxicillin - 1 g, bid | |
| Rifabutin - 150 mg, qd | |
| Culture-guided therapy (10-d) | PPI - standard dose, bid |
| Bismuth - standard dose, qid | |
| Two antibiotics selected by antimicrobial sensitivity tests |
First-line treatment may be split into two groups; treatment in areas with low clarithromycin resistance and in areas with high clarithromycin resistance. The most frequently used regimen in areas with low clarithromycin resistance is standard triple therapy while bismuth-containing quadruple therapy is also an alternative. The duration of therapy is suggested as 14 d by meta-analyses with eradication rates 5% higher than those with 7 d[13]. A Cochrane systemic review looked at 75 eligible studies and found that eradication rate was 83.5% for PPI, amoxicillin and clarithromycin; for PPI, clarithromycin and metronidazole the rate was 68.6% and for PPI, amoxicillin and metronidazole it was found to be 82%; each therapy lasting 14 d[14].
Increased dose of PPI, as strong suppression of acid secretion is essential for the stability and biological activity of antibiotics, or the increased length of treatment are factors improving the efficacy rates. A meta-analysis showed a greater beneficial effect with a double dose of esomeprazole while another meta-analysis showed lower cure rates in hosts who are extensive PPI metabolizers (depending on their cytochrome P450 status which PPI function relies on)[13]. Taking this into account, a study found out that administration of a PPI four times daily with amoxicillin or metronidazole in clarithromycin resistance, may result with eradication rate of 98%[15]. In a study done by Altintas et al[16] comparing the efficacy of different proton pump inhibitors - omeprazole, lansoprazole and pantoprazole - in combination with amoxicillin and clarithromycin in the first line eradication of H. pylori, there wasn’t any difference between the three groups[16]. Another meta-analysis done by Vergara et al[17] including fourteen studies found no difference between proton-pump inhibitors when used in standard triple therapy for H. pylori eradication. The in vitro antibacterial activity of proton pump inhibitors vary but still the eradication rates are similar which suggests that acid inhibition is the main antibacterial mechanism of proton-pump inhibitors in vivo[17]. On the other hand, although the eradication rate of triple therapy with clarithromycin fell to 65%, it remained as high as 84% with metronidazole[18]. It is mostly due to the fact that metronidazole resistance may be overcome by increasing the dose and prolonging treatment duration[19]. In areas with high clarithromycin resistance (i.e., Spain, Turkey, Alaska, China and Japan) bismuth quadruple, sequential, concomitant and hybrid therapies may be used[20].
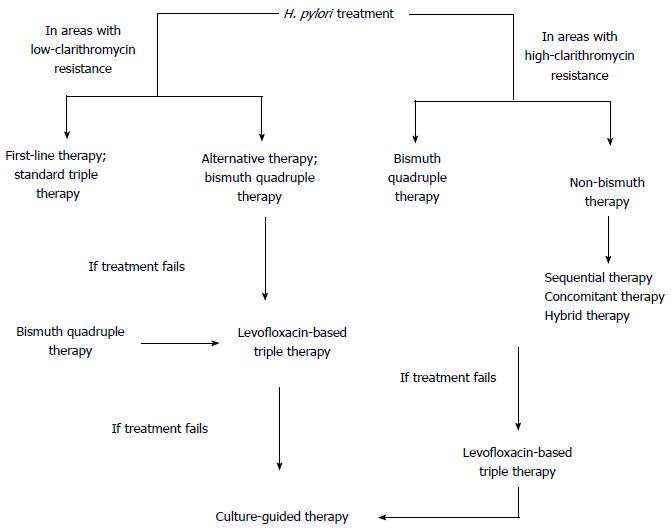
Bismuth quadruple therapy is recommended as a first-line therapy in areas with high clarithromycin resistance, as an alternative first-line therapy in areas with low clarithromycin resistance or as an empirical treatment when clarithromycin therapy fails (Figure 1)[21]. It involves combination of a PPI, bismuth subsalicylate, metronidazole and tetracycline for 10 to 14 d (Table 1). This treatment is ideal as a second-line therapy, as recommended by The Maastricht IV Consensus Report[22] and the Second Asia-Pacific Consensus Guidelines[23]. Since it doesn’t contain clarithromycin, compliance with the regimen is high and also metronidazole resistance in vitro does not affect the outcome significantly[19]. Salazar et al[24] reported an eradication rate greater than 95% with 14-d bismuth-quadruple therapy. A study from China, looking at the efficacy of bismuth quadruple therapy with lansoprazole as PPI and tetracycline/amoxicillin with metronidazole or furazolidone yielded eradication rates of 87.9%-95.2%; best outcome being the combination of lansoprazole, bismuth, amoxicillin and furazolidone[25].
Among the meta-analyses comparing standard triple therapy with bismuth quadruple therapy as first-line treatment, a study done by Venerito et al[26] showed eradication rate of 77.6% with bismuth quadruple therapy whereas it was 68.9% with clarithromycin-based standard therapy. A meta-analysis done by Luther et al[27] concluded that quadruple and triple therapies yielded similar eradication rates when applied as primary therapy for H. pylori infection and revealed similar side effects. In regions with high clarithromycin resistance, it is suggested as first-line therapy and achieved eradication rate of 82% compared to standard triple therapy[12]. Marin et al[28] reported eradication rates of 76%, 77% and 82% for 7, 10 and 14 d, respectively, with bismuth quadruple therapy when they applied bismuth quadruple therapy as rescue therapy. Also, Liang et al[25] declared eradication rates of > 90% in patients who did not respond to previous therapies, including those with metronidazole resistance.
As bismuth is concentrated in H. pylori and the organism doesn’t develop resistance to it, applying bismuth quadruple therapy is advantageous over non-bismuth therapy[7]. The main limitations of this therapy are non-availability of bismuth salts or tetracycline in some countries as well as potential toxicity of bismuth. Nevertheless, no differences in terms of tolerability were found between non-bismuth and bismuth containing therapies in a study among 4763 patients, except dark stools being more common in bismuth-containing group[29]. A single capsule formulation has been developed (Pylera) to overcome the complexity of quadruple therapy and showed good efficacy[20]. If the bismuth quadruple therapy is not available, sequential, concomitant or hybrid therapies may be administered.
Sequential therapy, proposed by a group of Italian researchers, is a novel treatment intending to administer the antimicrobials in sequence. It is a 10-d treatment consisting of 5 d of PPI therapy with amoxicillin followed by a further 5 d of PPI with clarithromycin and metronidazole (Table 1). The main goal of sequential therapy, which has shown to have success rate of 90%-94% in several studies, is to overcome clarithromycin resistance[20]. It has been deemed that administration of amoxicillin deteriorates the bacterial cell wall, which ends up transferring clarithromycin out of the bacteria by preventing the development of efflux channels[5]. A study from China showed that sequential therapy achieves significantly higher eradication rates than triple therapy in patients with clarithromycin-resistant strains; while neither treatment was good enough to reach an eradication rate higher than 55% when clarithromycin and metronidazole resistance exists[30]. Similar results were obtained in a study from Korea where a high prevalence of clarithromycin resistant H. pylori is seen; eradication rates of 79% and 62% with sequential and triple therapy, respectively[31]. One study from Turkey, where clarithromycin resistance is high, reported success rate of 78% with sequential therapy vs 53% with standard triple therapy[32]. However, a recent meta-analysis evaluating H. pylori eradication rates in children showed that although sequential therapy is superior to 7-d triple therapy, it is not significantly better than 10- or 14-d triple therapy[13]. Three meta-analyses that took place in Asia in 2014 comparing efficacy of sequential therapy with standard triple therapy, favored sequential therapy over standard therapy[33-35]. Also, in a meta-analysis done by Zullo et al[36] success rate of sequential regimen was found higher (approximately 10%) compared to the triple therapy. Beside the mentioned study and meta-analyses, in Latin America countries, studies showed that standard triple therapy for 14 d was superior to sequential therapy (82% vs 76.5% respectively). This conflicting data might be due to variations in the prevalence of antibiotic resistance, which are lower in Latin America countries[37].
Sequential therapy is also recommended as first-line therapy like bismuth quadruple therapy in areas with high-clarithromycin resistance, i.e., Italy and China with 90%-92% success rates[20]. In areas where bismuth drugs are not available, it may be necessary to prescribe sequential therapy[22]. A study done by Liu et al[38] reported that 10-d sequential and modified bismuth quadruple therapies are both highly effective as empirical first-line therapies in Chinese patients.
Concomitant therapy is proposed in order to reduce the complexity of sequential therapy which involves simultaneous administration of three antibiotics and a PPI for 10 d (Table 1). This treatment is used in areas where high-clarithromycin resistance is present and bismuth-based quadruple therapy is not available (Figure 1). When compared with standard triple therapy in meta-analyses of randomized trials concomitant therapy was found to be superior to standard triple therapy with 90% eradication rate[39]. In the presence of dual-resistance (100% clarithromycin and 91% metronidazole resistance), eradication rate was only 55% with concomitant therapy[5]. However, in a recent controlled trial done in Greece where clarithromycin resistance was 25% and metronidazole resistance was 40%, eradication rate was 90% under concomitant therapy[40]. Considering the sequential therapy, the eradication rates were found similar in a prospective randomized clinical trial in Spain; 91% with concomitant therapy and 86% with sequential therapy[41]. In addition, Gatta et al[42] found no superiority between sequential and concomitant therapies. If the concomitant therapy fails, empirical therapy becomes difficult due to exposure to both metronidazole and clarithromycin; as a result, a levofloxacin-containing or rifabutin-containing regimen may be necessary[7].
Hybrid (dual-concomitant) therapy, which consists of two steps; 7 d of PPI and amoxicillin followed by a PPI and clarithromycin, amoxicillin and metronidazole, intends to overcome resistance with the benefits of four drugs of the concomitant therapy (Table 1). This therapy was first described by Hsu et al[43] and eradication rate of 99% by per-protocol and 97.4% by intention-to-treat analysis were obtained in 117 treated patients. Studies done involving Spanish and Italian people showed similar eradication rates (approximately 90%) for both hybrid and concomitant therapies[42]. Comparing hybrid and sequential therapies, 89.5% and 76.7% success rates were reported in a study with similar severe adverse effects[13]. More studies are needed to understand the efficacy of hybrid therapy.
Levofloxacin-based therapy is recommended whenever bismuth quadruple therapy fails, in areas with both low and high clarithromycin resistance. It is a 10-d treatment with amoxicillin, levofloxacin and PPI (Table 1). Although eradication rate is around 90% when used instead of clarithromycin in either triple or sequential therapies, the obstacle to use levofloxacin as a first-line treatment is the increasing frequency of quinolone resistance; which is currently 40% in America, 20% in Europe and 10% in Asia[5,44]. The meta-analysis done among patients who failed eradication with standard triple therapy showed better eradication rates with levofloxacin triple therapy than bismuth quadruple therapy, 81% and 70% respectively[45]. Including levofloxacin instead of clarithromycin in sequential therapy showed higher eradication rates in a study done by Gatta et al[42] Moxifloxacin and sitafloxacin may also be used but there is no evidence supporting advantage over levofloxacin. It is better to reserve fluoroquinolones for use in rescue regimens when the therapy with clarithromycin or metronidazole treatment fails.
Another salvage therapy is with rifabutin (Table 1). The advantage is the low frequency of rifabutin resistance. In a study done in Korea, where high prevalence of levofloxacin resistance is seen, amongst patients who had failed two initial regimens rifabutin triple therapy had better eradication rates than levofloxacin triple therapy, 71% and 57% respectively[46]. 50% success rate was achieved by rifabutin-based therapy in patients who had failed clarithromycin-, metronidazole- and levofloxacin-based therapy[47].
The Maastricht IV Consensus Report recommends antimicrobial susceptibility testing when the second-line treatment fails[22]. Although it will provide the best choice of antibiotics that can be used, the sensitivity of culture has been reported as < 60%[18]. In a study done by Cammarota et al[48], 90% of eradication rate was obtained among patients treated with a culture-guided third-line regimen. Culture-guided therapy is a 10-d quadruple therapy comprising a PPI, bismuth and two antibiotics selected by antimicrobial sensitivity tests (Table 1).
There are multiple approaches identified to overcome the side effects and increase the efficacy of treatment in H. pylori infections. Nowadays, use of probiotics is an emerging treatment option. They improve the eradication rates and side effects of the therapies used in H. pylori treatment by reducing H. pylori adhesion or colonization[49]. While a meta-analysis evaluating probiotics found increase in eradication rates with both Lactobacillus and Bifidobacterium, no significant improvement in side effects was seen[50]. A meta-analysis of nine studies on probiotic use as an adjuvant therapy found raise in eradication rates by 17%[51]. When Saccharomyces boulardii was added to H. pylori eradication regimens, decrease in side effects, especially in diarrhea, was observed as well as higher eradication rates[50,52]. Since the safety profile of probiotics is known, it is reasonable to suggest people under H. pylori therapy to eat yogurt.
Pre-treatment with n-acetlycysteine as a mucolytic agent is another approach to destroy the biofilm of H. pylori and overcome the antibiotic resistance[53]. In a randomized controlled trial done amongst patients with a history of at least four eradication failures, the eradication rates were found higher in the group who received n-acetylcysteine before a culture-guided regimen[54].
Simvastatin was used in a randomized controlled trial where the proposed mechanism of action was its anti-inflammatory effect other than its cholesterol lowering effect and the eradication rates were found to be increased but no improvement was noted in side effects[55].
The studies on vaccine against H. pylori still continue. A recent vaccine based on Cag A - Vac A - neutrophil-activating proteins was developed but although recognized by the host’s cellular and humoral immune systems, limited immunogenicity was observed[56]. Altman et al[57] modulated H. pylori lipopolysaccharides chemically to enhance immunogenicity which enhanced antibody responses and a modest reduction in gastric H. pylori load when administered prophylactically. Still, there is no vaccine in use.
H. pylori is associated with multiple diseases and 100% eradication is still not possible. Even after a successful eradication, reinfection or recurrence can occur. The efficacy of standard triple therapy is decreasing whilst the bismuth quadruple and sequential regimen has been proven to achieve higher cure rates. Even though there are multiple guidelines (all therapies are summarized in Table 1) about the treatment regimens, increasing antibiotic resistance as well as different frequencies in resistance in different areas of the world suggests the optimal approach in the treatment of patients with H. pylori infections to be individualized therapy, which is a highly active and well-tolerated regimen. Use of probiotics, pre-treatment with n-acetylcysteine and statins may help as adjuvant therapies.
P- Reviewer: Kurtoglu E, Lee CL, Nakajima N S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:1-8. [PubMed] [Cited in This Article: ] |
| 2. | Zhao B, Zhao J, Cheng WF, Shi WJ, Liu W, Pan XL, Zhang GX. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, Provan D, Newland A, Amadori S, Bussel JB. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113:1231-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Bazzoli F, Pozzato P. Therapy of H. pylori infection. J Physiol Pharmacol. 1997;48 Suppl 4:39-46. [PubMed] [Cited in This Article: ] |
| 5. | Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol. 2014;5:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Lee JY, Kim N, Kim MS, Choi YJ, Lee JW, Yoon H, Shin CM, Park YS, Lee DH, Jung HC. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59:1235-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Vakil N, Vaira D. Treatment for H. pylori infection: new challenges with antimicrobial resistance. J Clin Gastroenterol. 2013;47:383-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Wu W, Yang Y, Sun G. Recent Insights into Antibiotic Resistance in Helicobacter pylori Eradication. Gastroenterol Res Pract. 2012;2012:723183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 694] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 10. | Smith SM. Role of Toll-like receptors in Helicobacter pylori infection and immunity. World J Gastrointest Pathophysiol. 2014;5:133-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 66] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 607] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 12. | Cizginer S, Ordulu Z, Kadayifci A. Approach to Helicobacter pylori infection in geriatric population. World J Gastrointest Pharmacol Ther. 2014;5:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol. 2014;20:1438-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 119] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (2)] |
| 14. | Yuan Y, Ford AC, Khan KJ, Gisbert JP, Forman D, Leontiadis GI, Tse F, Calvet X, Fallone C, Fischbach L. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Malfertheiner P, Selgrad M. Helicobacter pylori. Curr Opin Gastroenterol. 2014;30:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Altintas E, Sezgin O, Ulu O, Aydin O, Camdeviren H. Maastricht II treatment scheme and efficacy of different proton pump inhibitors in eradicating Helicobacter pylori. World J Gastroenterol. 2004;10:1656-1658. [PubMed] [Cited in This Article: ] |
| 17. | Vergara M, Vallve M, Gisbert JP, Calvet X. Meta-analysis: comparative efficacy of different proton-pump inhibitors in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;18:647-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Sasaki H, Nagahara A, Hojo M, Asaoka D, Matsumoto K, Osada T, Watanabe S. Ten-year trend of the cumulative Helicobacter pylori eradication rate for the ‘Japanese eradication strategy’. Digestion. 2013;88:272-278. [PubMed] [Cited in This Article: ] |
| 19. | Graham DY, Qureshi WA. Antibiotic-resistant H. pylori infection and its treatment. Curr Pharm Des. 2000;6:1537-1544. [PubMed] [Cited in This Article: ] |
| 20. | Urgesi R, Cianci R, Riccioni ME. Update on triple therapy for eradication of Helicobacter pylori: current status of the art. Clin Exp Gastroenterol. 2012;5:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Furuta T, Sugimoto M, Shirai N, Matsushita F, Nakajima H, Kumagai J, Senoo K, Kodaira C, Nishino M, Yamade M. Effect of MDR1 C3435T polymorphism on cure rates of Helicobacter pylori infection by triple therapy with lansoprazole, amoxicillin and clarithromycin in relation to CYP 2C19 genotypes and 23S rRNA genotypes of H. pylori. Aliment Pharmacol Ther. 2007;26:693-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1494] [Article Influence: 124.5] [Reference Citation Analysis (3)] |
| 23. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 24. | Salazar CO, Cardenas VM, Reddy RK, Dominguez DC, Snyder LK, Graham DY. Greater than 95% success with 14-day bismuth quadruple anti- Helicobacter pylori therapy: a pilot study in US Hispanics. Helicobacter. 2012;17:382-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, Xiao S, Lu H. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013;11:802-807.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013;14:843-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol. 2008;14:7361-7370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 85] [Cited by in F6Publishing: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Zhou L, Zhang J, Chen M, Hou X, Li Z, Song Z, He L, Lin S. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. Am J Gastroenterol. 2014;109:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, Lee JH, Yang CH, Kim ES, Cho KB. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012;35:56-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 32. | Nadir I, Yonem O, Ozin Y, Kilic ZM, Sezgin O. Comparison of two different treatment protocols in Helicobacter pylori eradication. South Med J. 2011;104:102-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Yoon H, Lee DH, Kim N, Park YS, Shin CM, Kang KK, Oh DH, Jang DK, Chung JW. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013;28:1801-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Kim JS, Ji JS, Choi H, Kim JH. Sequential therapy or triple therapy for Helicobacter pylori infection in Asians: systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:118-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Chung JW, Ha M, Yun SC, Kim JH, Lee JJ, Kim YJ, Kim KO, Kwon KA, Park DK, Lee DH. Meta-analysis: Sequential therapy is superior to conventional therapy for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2013;62:267-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Zullo A, Hassan C, Ridola L, De Francesco V, Vaira D. Standard triple and sequential therapies for Helicobacter pylori eradication: an update. Eur J Intern Med. 2013;24:16-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 38. | Liu KS, Hung IF, Seto WK, Tong T, Hsu AS, Lam FY, But DY, Wong SY, Leung WK. Ten day sequential versus 10 day modified bismuth quadruple therapy as empirical firstline and secondline treatment for Helicobacter pylori in Chinese patients: an open label, randomised, crossover trial. Gut. 2014;63:1410-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 40. | McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 41. | McNicholl AG, Marin AC, Molina-Infante J, Castro M, Barrio J, Ducons J, Calvet X, de la Coba C, Montoro M, Bory F. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014;63:244-249. [PubMed] [Cited in This Article: ] |
| 42. | Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 193] [Article Influence: 17.5] [Reference Citation Analysis (95)] |
| 43. | Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 44. | De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409-414. [PubMed] [Cited in This Article: ] |
| 45. | Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101:488-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 46. | Jeong MH, Chung JW, Lee SJ, Ha M, Jeong SH, Na S, Na BS, Park SK, Kim YJ, Kwon KA. Comparison of rifabutin- and levofloxacin-based third-line rescue therapies for Helicobacter pylori. Korean J Gastroenterol. 2012;59:401-406. [PubMed] [Cited in This Article: ] |
| 47. | Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35:941-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Cammarota G, Martino A, Pirozzi G, Cianci R, Branca G, Nista EC, Cazzato A, Cannizzaro O, Miele L, Grieco A. High efficacy of 1-week doxycycline- and amoxicillin-based quadruple regimen in a culture-guided, third-line treatment approach for Helicobacter pylori infection. Aliment Pharmacol Ther. 2004;19:789-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Ruggiero P. Use of probiotics in the fight against Helicobacter pylori. World J Gastrointest Pathophysiol. 2014;5:384-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 54] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol. 2014;20:12781-12808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 188] [Cited by in F6Publishing: 188] [Article Influence: 18.8] [Reference Citation Analysis (2)] |
| 51. | Zheng X, Lyu L, Mei Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: evidence from a meta-analysis. Rev Esp Enferm Dig. 2013;105:445-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Song MJ, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010;15:206-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Malfertheiner P, Selgrad M, Bornschein J. Helicobacter pylori: clinical management. Curr Opin Gastroenterol. 2012;28:608-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Cammarota G, Branca G, Ardito F, Sanguinetti M, Ianiro G, Cianci R, Torelli R, Masala G, Gasbarrini A, Fadda G. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clin Gastroenterol Hepatol. 2010;8:817-820.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Malfertheiner P, Venerito M, Selgrad M. Helicobacter pylori infection: selected aspects in clinical management. Curr Opin Gastroenterol. 2013;29:669-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Ruggiero P. Helicobacter pylori infection: what’s new. Curr Opin Infect Dis. 2012;25:337-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Altman E, Chandan V, Harrison BA, Veloso-Pita R, Li J, KuoLee R, Chen W, Vérez-Bencomo V; Regional Helicobacter pylori Study Group. Design and immunological properties of Helicobacter pylori glycoconjugates based on a truncated lipopolysaccharide lacking Lewis antigen and comprising an α-1,6-glucan chain. Vaccine. 2012;30:7332-7341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |