Copyright
©2012 Baishideng.
World J Methodol. Oct 26, 2012; 2(5): 33-41
Published online Oct 26, 2012. doi: 10.5662/wjm.v2.i5.33
Published online Oct 26, 2012. doi: 10.5662/wjm.v2.i5.33
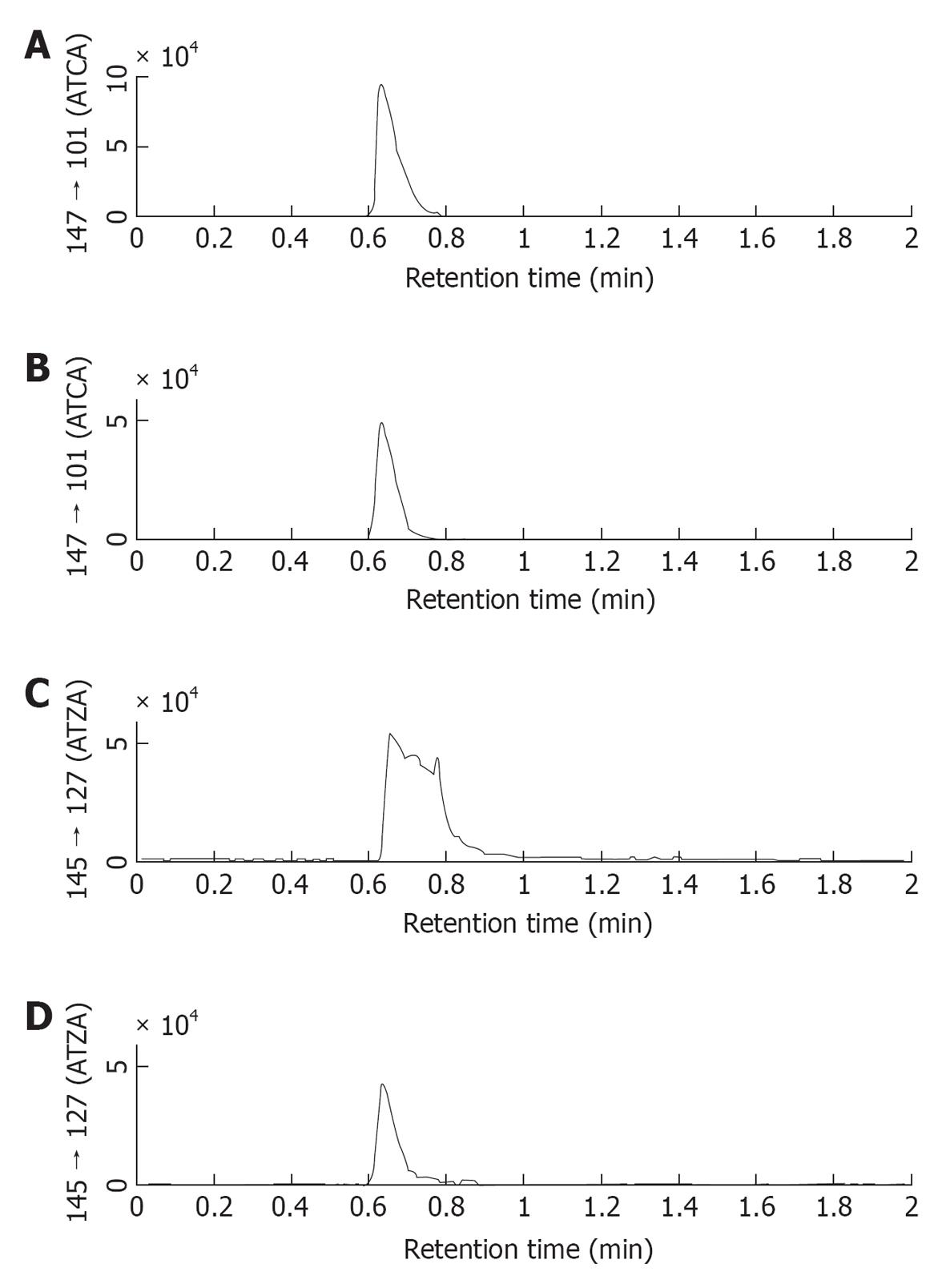
Figure 3 Comparison of chromatograms of 2-aminothiazoline-4-carboxylic acid and 2-aminothiazole-4-carboxylic acid obtained by using 0.
5% acetic acid/MeOH as mobile phase (A and C) and 0.5% trifluoroacetic acid/MeOH as mobile phases (B and D). When trifluoroacetic acid (TFA) was added to the mobile phase a good sharp 2-aminothiazole-4-carboxylic acid (ATZA) peak was detected. This result suggested that TFA was a more effective ion-paring reagent than acetic acid during the chromatography. Therefore, 0.5% TFA/MeOH was selected as the mobile phase, replacing the 0.5% acetic acid/MeOH mobile phase used in our prior work. ATCA: 2-aminothiazoline-4-carboxylic acid.
- Citation: Yu JC, Martin S, Nasr J, Stafford K, Thompson D, Petrikovics I. LC-MS/MS analysis of 2-aminothiazoline-4-carboxylic acid as a forensic biomarker for cyanide poisoning. World J Methodol 2012; 2(5): 33-41
- URL: https://www.wjgnet.com/2222-0682/full/v2/i5/33.htm
- DOI: https://dx.doi.org/10.5662/wjm.v2.i5.33