Published online Apr 12, 2017. doi: 10.5528/wjtm.v6.i1.1
Peer-review started: October 31, 2016
First decision: February 16, 2017
Revised: February 23, 2017
Accepted: March 13, 2017
Article in press: March 15, 2017
Published online: April 12, 2017
The ubiquitousness of naturally occurring boron-containing compounds (BCCs) has led to their constant contact with humankind. Recently, many synthetic BCCs have been elaborated for a broad spectrum of purposes, especially boric, boronic and borinic acids. Although BCCs were once employed primarily as antiseptics and later as antibiotics, they have become an increasingly relevant therapeutic tool. Nevertheless, this potential of BCCs has been drastically limited due to some unfortunate intra-hospital accidents in the 1940s and 1950s. The increasing use of BCCs as insecticides, antimicrobials, and other agents is providing new insights into their role in the physiology of several living species and in the pathophysiology of humans. It is becoming clear that BCCs act through a wide range of mechanisms, as do their corresponding boron-free counterparts. When comparing BCCs and similar boron-free compounds, in many cases the former show advantages in the medical field. The current mini-review focuses on how BCCs have been developed by means of translational medicine, a process connecting biomedical research with clinical applications. This process of discovery is currently in an exponential stage.
Core tip: We herein review the scope of boron-containing compounds (BCCs) with therapeutic applications. Information is compiled about the physiological relevance of boron, as well as the medicinal use of BCCs. Apart from describing BCCs employed to treat neoplastic and infectious disease, mention is made of such compounds that may have therapeutic potential for other maladies.
- Citation: García-Ávila AK, Farfán-García ED, Guevara-Salazar JA, Trujillo-Ferrara JG, Soriano-Ursúa MA. Scope of translational medicine in developing boron-containing compounds for therapeutics. World J Transl Med 2017; 6(1): 1-9
- URL: https://www.wjgnet.com/2220-6132/full/v6/i1/1.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v6.i1.1
Due to the ubiquitousness of naturally occurring boron-containing compounds (BCCs), they have been in contact with humankind since prehistoric times[1]. Some researchers suggest that contact between BCCs and living beings dates to the origin of life, and others that BCCs gave the proper conditions for the generation of primitive nucleic acids in the ancient oceans of the planet[2].
Described as an element in 1824 by Berzelius[3], boron has a mass of 10.81 and is the fifth element of the periodic table. This element is defined as a metalloid and semiconductor. Despite being scarce in the universe, it is relatively abundant in the earth’s crust, having an estimated concentration of about 10 ppm and a mass of about 2.4 × 1017 kg. This represents 10 times the concentration found in some meteorites (where boron has the second greatest presence in the solar system) and one million times the concentration on other planets[4].
The estimated concentration of boron is 4.6 ppm in seawater, where it is a component of two hydrated molecules: B(OH)3 trigonal (resembling the tetrahedral configuration of carbon) and B(OH)4 tetrahedral. The proportion of the two forms depends on the local pH of seawater, given that basic environments favor the tetrahedral form and acid environments the trigonal form. Boron reaches the atmosphere through the evaporation of seawater, and then returns to the ocean or falls on continental landmasses by precipitation[4]. There are two naturally occurring isotopes of boron, 11B (80.1%) and 10B (19.9%), and eleven other known isotopes.
Some synthetic BCCs are commonplace today, especially boric, boronic and borinic acids used to produce textiles, detergents, glass, semi-conductors, and materials for protecting against radiation. Moreover, boron fibers are employed for configurational mechanics, amorphous boron for pyrotechnics, and boron atoms for the detection of neutrons in nuclear reactors. Research has been conducted on borohydrides to generate fuels[1] and on some BCCs to diagnose and/or cure disease, although to date only a few of the latter compounds have been administered to patients.
Historically speaking, the development of BCCs for therapeutic purposes has been slow. To preserve bodies in the process of mummification, the Egyptians made a blend of salts (including borates) called natron[5]. Shortly afterwards, borosilicate became an element of glass manufacturing and pottery making. Blends of boronic acids and other salts were utilized in wood finishing, which apparently gave rise to the application of boron salts as a cataplasm for exposed wounds.
Boric and boronic acids were later administered as a prophylactic treatment to prevent infectious events and the propagation of disease, a practice continued into the XX century[1]. It was reported in the 1900s that boron could be beneficial in plague control due to its toxicity for several insects (e.g., termites, ants and cockroaches) that can damage crops or infrastructure, or transmit disease. However, little was known about the mechanisms by which boron causes those effects. While BCCs are being used to treat sexually transmitted diseases and other topical infections[6,7], investigation continues into the nature of their antimicrobial activity. At the beginning of the XVIII century, boron in the form of boracic acid, called “sal sedativum Hombergi”, was proposed as a sedative or analgesic agent, but without much success[1,8].
Because of the cytotoxic properties of boron, in the XX century it began to be included in some antineoplastic drugs. The most common of these is a proteasome inhibitor called bortezomib, a drug successfully given to patients suffering multiple myeloma[9]. Other BCCs have been studied for antineoplastic activity, but the only real advance in this context has been the discovery of new targets for malignancies[10,11]. In the technique denominated boron neutron capture therapy, compounds containing the B isotope (which is not radioactive) are utilized to control local invasive tumors[12].
As can be appreciated, BCCs have gradually become a relevant medicinal tool. Nevertheless, the potential of BCCs in therapeutics and diagnosis has been drastically limited due to some unfortunate hospital accidents in the 1940s and 1950s. These were cases of the misuse of boric acid that resulted in illness and even death. In the worst of cases, some infants were mistakenly given high doses of this weak acid (which was at that time employed as a nosocomial antisepsis) instead of powdered infant formula[13,14]. Multiple studies have proven that the fear of administering BCCs is groundless. Boron has different effects on organisms at distinct levels in the phylogenetic scale, producing greater toxicity in prokaryotes, protozoaries and insects than in mammals. Moreover, the toxicity induced by BCCs is governed by the same structure-activity relation that holds true for boron-free compounds[15]. There are BCCs with high and low toxicity as well as with high and low potency to modify the activity of a biological entity. Accordingly, research on BCCs has confirmed the observation of Paracelsus: “The dose makes the venom”. In other words, the aim in developing BCCs and all other medicinal compounds is to cause a certain biological effect while avoiding toxicity, based on the pharmacodynamics and pharmacokinetics of the agent in question[16]. Translational medicine has been applied to achieve this goal, enabling the introduction of BCCs into the clinical setting in an accelerated timeframe by identifying compounds with great therapeutic potential.
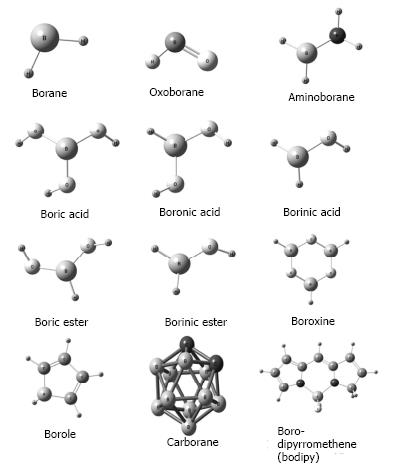
Nowadays the number of BCCs has increased exponentially because of the development of new synthetic compounds included in materials, drugs and other uses[8,16]. In the biological area, of particular interest are boranes, oxyborates, aminoboranes, carboranes and boron-dipyrromethenes (Figure 1).
The relevance of BCCs in the physiology of plants is well known. On the other hand, since only a scant intake is necessary for the basic functions of mammals, the understanding of the physiological role of this element is more complicated. Whereas a greater quantity of boron is provided by the consumption of vegetables than animal products, either one of these source fulfills the needs of the organism of humans and other mammals[17,18]. Experimental models have shown an essential role for this element in mammals. Guidelines by several nations establish a minimum daily intake of 0.25 mg for humans, easily acquired from food. In fact, it is estimated that the average American diet furnishes 1-2 mg per day. A diet with over 3.25 mg per day is considered high in boron[19].
A boron deficiency in the mammalian diet can influence several functions, the most relevant of which are the modulation of bone growth, the immunological response, and some mental functions[20,21]. It is well documented that BCCs influence enzymatic activity, the metabolism of steroid hormones, and the metabolism and activity of other micronutrients such as calcium and magnesium. Therefore, some BCCs could possibly be used as therapeutic tools for arthritis, metabolic diseases, central nervous system disorders and various infectious diseases[21].
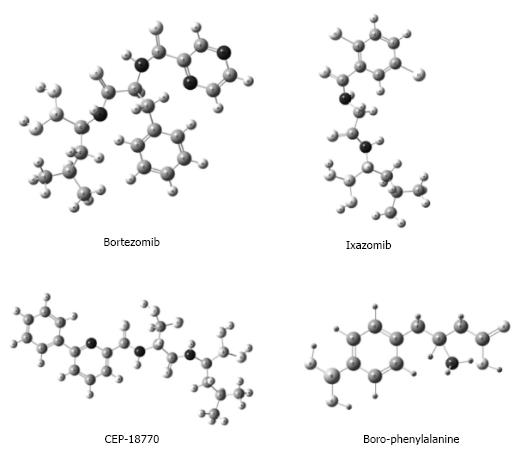
Bortezomib is a product of translational medicine. It was developed in 1995 by Myogenics company, which changed its name to ProScript, and later was bought by Millennium Pharmaceuticals in October, 1999. This drug is representative of boron-containing proteasome inhibitors, which are enzyme complexes that regulate protein homeostasis within the cell (Figure 2). Bortezomib was tested in a small Phase I clinical trial on multiple myeloma patients after promising preclinical results. One of the first patients to receive the drug in a clinical trial achieved a complete response and was still alive four years later. This led to subsequent clinical trials that indicated the likelihood of a complete response in fifteen percent of patients. In 2003, Bortezomib was approved by United States-Food and Drug Administration (FDA) for the treatment of multiple myeloma[22,23]. Since then, the use of this BCC has grown exponentially to include the treatment of Waldenström’s macroglobulinemia[24,25], non-Hodgkin’s lymphoma[26], antibody-mediated rejection in cardiac and kidney transplantation[27,28], cutaneous T-cell lymphomas (mycosis fungoides)[29], relapsed or refractory follicular lymphoma[30], peripheral T-cell lymphoma and relapsed systemic light-chain amyloidosis[31,32].
Bortezomib reversibly inhibits chymotrypsin-like activity at the 26S proteasome, leading to activation of signaling cascades, cell-cycle arrest and apoptosis[22], which makes it suitable as an antineoplastic agent. It can be administered intravenously or subcutaneously (SC), but the latter route is generally preferred due to a lower risk of adverse effects, mainly neuropathy[33].
Regarding carcinogenesis, bortezomib is utilized primarily as a component of therapeutic regimes for the treatment of multiple myeloma, whether in newly diagnosed, relapse or refractory cases, due to the high rates of positive response and good tolerability. In this sense, the most common regimen is bortezomib, lenalidomide and dexamethasone (VRd)[34,35], which is a particularly advantageous combination for patients at risk, such as those with renal failure. Other combinations employed are: Bortezomib, cyclophosphamide and dexamethasone (VCd); bortezomib, thalidomide and dexamethasone (VTd); and bortezomib plus dexamethasone (VD). A high rate of positive response has been proven for all these combinations in several trials, including SWOG S0777[35] and IFM2013-04[36].
Bortezomib is not free from adverse effects, the most common of which is peripheral neuropathy. Others are anorexia, nausea and vomiting, cutaneous reactions, neutropenia, and thrombocytopenia[37,38]. Although the latter occurs in 43% of patients, it is rarely severe enough to postpone subsequent cycles[39]. Peripheral neuropathy, often painful, develops in approximately 40% of patients who receive bortezomib 1.3 mg/m2 twice weekly[40,41] and in about 20% of patients who receive the same dose once weekly[42]. It appears to be less frequent and less severe when the drug is administered subcutaneously[33]. However, it is more frequent and more severe in patients who previously received neurotoxic therapy as well as in those with preexisting neuropathy[43]. Given the high rate of neuropathy, a dose adjustment algorithm is available in the full prescription information at the FDA website. Bortezomib therapy may also be associated with an increased risk of herpes zoster[44].
Overall, bortezomib is an excellent example of how BCCs are like any drug in the medical field. The toxicity induced by BCCs is governed by the same structure-activity relation that holds true for boron-free compounds, and the mechanisms of action of BCCs are in most cases similar to those of the corresponding boron-free compounds. As new insights into the action mechanism of BCCs are provided, new applications will certainly be found.
There are currently two other boronic acid-based proteasome inhibitors that have been tested in a clinical trial. Ixazomib (2, MLN9708)[45] is the first oral proteasome inhibitor that has managed to receive a positive opinion (on September 16th of 2016) by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA). The EMA recommended the conditional approval of NINLAROTM (ixazomib) capsules combined with lenalidomide and dexamethasone for the treatment of adult patients suffering from multiple myeloma as long as they have received at least one prior therapy. If the EMA ratifies the CHMP’s opinion and grants authorization, NINLARO will be the first and only oral proteasome inhibitor approved for use across the European Economic Area[46]. Another compound being studied is CEP-18770, which exhibited good anti-tumor activities against multiple myeloma in a mouse xenograft model and has entered a Phase I clinical trial[45].
Research based on translational medicine tends to accelerate the process by which compounds reach clinical practice. One of the attractive features of BCCs from a medicinal chemistry perspective is the tetrahedral geometry acquired by boron under conditions of physiological pH. It can thus mimic a tetrahedral carbon and the tetrahedral transition state of the enzyme-catalyzed substrate. Hence, BCCs are attractive biological agents in the pharmacological field[46].
Apart from the cases of the boron-containing proteasome inhibitors (bortezomib and ixazomib) to treat multiple myeloma, scientists have developed inhibitors of serine proteases that are responsible for coordinating various physiological functions such as blood coagulation, digestion, reproduction and immune response[47]. Boronic acids work as potent serine protease inhibitors by mimicking the tetrahedral transition state of peptide-bond hydrolysis[46]. As a result, BCCs are employed in the medical field to inhibit thrombin, lactamase, NS3 serine protease and DPP-IV, as well as to counter antibiotic resistance (because of their effect on beta lactamases) and treat hypercoagulability states, hepatitis C virus infection, diabetes, fibrotic diseases, angiogenesis, chronic inflammation and tumor progression[45,46].
Not only has there been a notable rise in the number of BCCs, but also in the knowledge of their importance in human physiology and physiopathology. For some of these compounds, the increasing data about their characteristics and action mechanisms indicate that they induce an advantageous biological response compared to the corresponding boron-free compounds[16]. On the other hand, multiple studies have shown a similar toxicological profile in mammals for BCCs and their boron-free counterparts, thus sparking new interest in designing and testing the former[15,48,49].
The growing knowledge about the mechanism by which BCCs act to provoke death and infertility in insects has enabled the development of new compounds for vector control that result in a lesser ecological impact than those used previously. That is, the new BCCs produce less damage to the non-targeted species[50,51].
Regarding prokaryotes, it is known that their genetic information is severely altered by BCCs. Some researchers even consider these compounds to be a new class of gene toxin[52]. The genotoxicity of BCCs causes rapid damage and could be lethal for microorganisms. However, the same is not observed in eukaryote cells, where genotoxic trials suggest only slight toxicity at concentrations greater than 1 mmol/L[53]. Hence, BCCs are highly selective in generating toxicity between species.
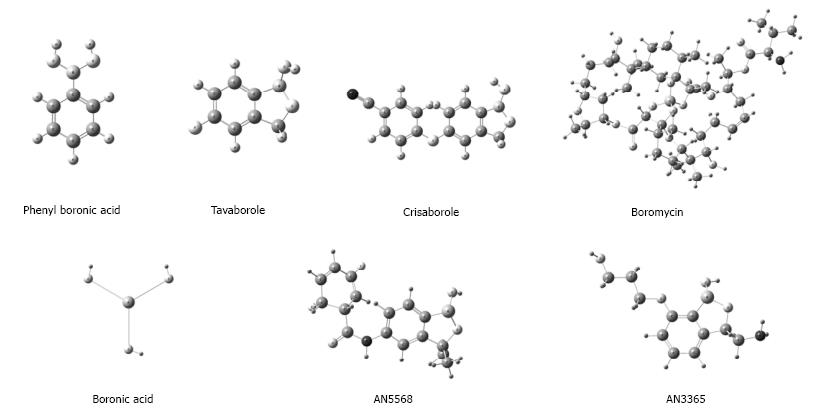
Little is known about the pharmacodynamics and molecular mechanisms by which BCCs bring about genotoxicity or the disturbance of other cellular processes (e.g., enzymatic functions). These compounds are frequently found in stable interaction with lateral chains of amino acids, bringing about the exposure of hydroxyls (serine, threonine and tyrosine) or the activation of autophagosomes[16,54]. This phenomenon has been explored in the last few years, affording attractive antibiotics (possibly also additional antineoplastic agents) by modifying naturally occurring BCCs with an antibiotic effect (like boromycin) or creating structural analogues of compounds showing microbicidal action (e.g., BCC analogues of beta-lactam) (Figure 3)[54-56].
Investigation into the selectivity of BCCs for microorganisms has also revealed the impact of these compounds on eukaryotes, leading to their proposed use in cancer treatment. The effectiveness of BCCs as therapy for some common diseases has encouraged the growth of business and government investment in further research. This tendency is evidenced by the growth of the pharmaceutical company ANACOR (a specialist in the design and testing of drugs that contain boron) and the interest of large pharmaceutical companies (e.g., Glaxo-Smith-Kline, Novartis and Lilly) and university research groups in the development of potential boron-containing drugs[57].
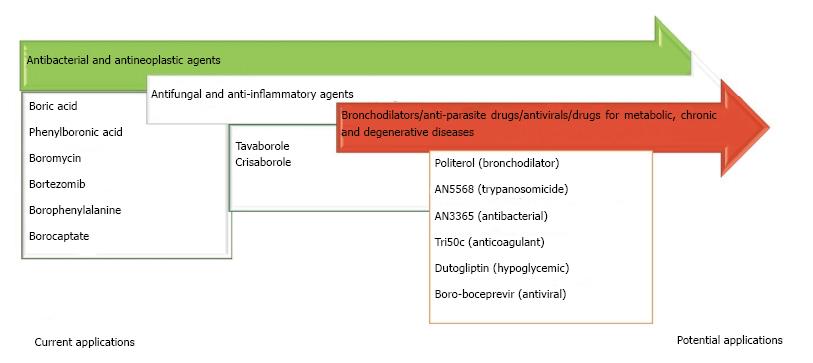
Though an increase in antimicrobials has been favored by the intense study of BCCs, the treatment of diverse pathologies with these compounds could take a couple decades. Recently, reports on experimental models of disease have indicated that BCCs may positively affect various malignancies as well as cardiovascular, pulmonary, metabolic and central nervous system disorders, which would imply benefits to millions of patients (Figure 2)[11,16]. Interestingly, the mechanisms by which BCCs and their corresponding boron-free compounds carry out their activity are not very different[16,58]. Although the pharmacodynamics of BCCs is being gradually elucidated, less is known about their toxicology and pharmacokinetics[48,49]. It seems that the variability of BCCs is as great as that of boron-free compounds, meaning that it is necessary to generate and analyze data for each compound. For some naturally occurring BCCs (e.g., boric acid), there is dose-dependent toxicity and a wide therapeutic index. Moreover, proteins exist for the transport of these compounds through the cellular membranes of plants and the complex structures of organisms. Less is known about BCCs in animals, but it appears that no enzymes can hydrolyze the boron-oxygen or boron-carbon bonds found in these molecules[16,59]. Insights into the distribution and excretion of boric and phenylboronic acids in animals suggests that their kinetics is similar between species. Furthermore, like boron-free compounds, the kinetics of BCCs in mammals seems to vary according to the kinetic-structure relation[48,60,61]. More detailed information on the kinetics of these compounds is direly needed because the lack of clarity in this sense represents a great barrier to their topical administration. This limitation restricts the use of BCCs to conditions requiring a wide distribution, such as disseminated infections and malignancies[62].
Finally, BCCs have not only been elaborated as agents that act directly on biological targets (Figure 4), but also for biomaterials or systems of drug delivery (i.e., nanostructures) (Figure 5)[51]. Translational medicine has been applied to a lesser extent for the latter purpose.
Humankind has had contact with BCCs since the origin of the species. Perhaps this contact was even determinant for human origin[63]. However, the nature of many of the interactions between these compounds and the human organism are unknown or only recently suggested. Because limited knowledge about the toxicology and pharmacokinetics of boric acid caused some unfortunate accidents 70-80 years ago, the study and especially the administration of BCCs has been restricted for many years. Nowadays, there is new enthusiasm and initiative to advance in this area for the solution of health problems. It is increasingly evident that BCCs act through similar mechanisms as their corresponding boron-free counterparts. The successful cases of developing therapeutic BCCs, often by means of translational medicine, has slowly yielded greater information about the pharmacodynamics of these compounds. Once restricted to anesthetics and antibiotics, the ever-greater insights in this field are opening new possibilities for treating cancer and other pathologies that impact the world population.
The authors thank the Secretaria de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-1754 y 20170411), the COFAA-IPN and the National Council of Science and Technology (CONACYT CB-235785) for the support given to the projects that are involved in the design and evaluation of boron-containing compounds.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: México
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Xu CS S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Woods WG. An introduction to boron: history, sources, uses, and chemistry. Environ Health Perspect. 1994;102 Suppl 7:5-11. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Neveu M, Kim HJ, Benner SA. The “strong” RNA world hypothesis: fifty years old. Astrobiology. 2013;13:391-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Berzelius JJ. Untersuchungen über die Flußspathsäure und deren merkwürdigste Verbindungen. Poggendorff’s Annalen der Physik und Chemie. 1824;78:113-150. [DOI] [Cited in This Article: ] |
| 4. | Kot FS. Boron sources, speciation and its potential impact on health. Rev Environ Sci Bio. 2009;8:3-28. [DOI] [Cited in This Article: ] |
| 5. | Garrett DE. Borates: handbook of deposits, processing, properties, and use. United Kingdom: Academic Press 1998; 385-386. [Cited in This Article: ] |
| 6. | Mendling W, Brasch J, Cornely OA, Effendy I, Friese K, Ginter-Hanselmayer G, Hof H, Mayser P, Mylonas I, Ruhnke M. Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses. 2015;58 Suppl 1:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Brittingham A, Wilson WA. The antimicrobial effect of boric acid on Trichomonas vaginalis. Sex Transm Dis. 2014;41:718-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | de Crell L. On the Decomposition of the Acid of Borax or Sedative Salt. Philos T R Soc B. 1799;89:56-73. [Cited in This Article: ] |
| 9. | Kouroukis TC, Baldassarre FG, Haynes AE, Imrie K, Reece DE, Cheung MC. Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol. 2014;21:e573-e603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Scorei RI, Popa R. Boron-containing compounds as preventive and chemotherapeutic agents for cancer. Anticancer Agents Med Chem. 2010;10:346-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Das BC, Thapa P, Karki R, Schinke C, Das S, Kambhampati S, Banerjee SK, Van Veldhuizen P, Verma A, Weiss LM. Boron chemicals in diagnosis and therapeutics. Future Med Chem. 2013;5:653-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Moss RL. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl Radiat Isot. 2014;88:2-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Grand RS, Wegner ES. Fatal case of boric acid poisoning. Am J Dis Child. 1948;75:910-912. [PubMed] [Cited in This Article: ] |
| 14. | Bean EO. Accidental ingestion of boric acid. Clin Proc Child Hosp Dist Columbia. 1949;5:101-103. [PubMed] [Cited in This Article: ] |
| 15. | Soriano-Ursúa MA, Farfán-García ED, López-Cabrera Y, Querejeta E, Trujillo-Ferrara JG. Boron-containing acids: preliminary evaluation of acute toxicity and access to the brain determined by Raman scattering spectroscopy. Neurotoxicology. 2014;40:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Soriano-Ursúa MA, Das BC, Trujillo-Ferrara JG. Boron-containing compounds: chemico-biological properties and expanding medicinal potential in prevention, diagnosis and therapy. Expert Opin Ther Pat. 2014;24:485-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Miwa K, Fujiwara T. Boron transport in plants: co-ordinated regulation of transporters. Ann Bot. 2010;105:1103-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Takano J, Miwa K, Fujiwara T. Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci. 2008;13:451-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Pizzorno L. Nothing Boring About Boron. Integr Med (Encinitas). 2015;14:35-48. [PubMed] [Cited in This Article: ] |
| 20. | Nielsen FH. Is boron nutritionally relevant? Nutr Rev. 2008;66:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Devirian TA, Volpe SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003;43:219-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Bladé J, Cibeira MT, Rosiñol L. Bortezomib: a valuable new antineoplastic strategy in multiple myeloma. Acta Oncol. 2005;44:440-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Brett LK, Williams ME. Current and emerging therapies in mantle cell lymphoma. Curr Treat Options Oncol. 2013;14:198-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Chen CI, Kouroukis CT, White D, Voralia M, Stadtmauer E, Stewart AK, Wright JJ, Powers J, Walsh W, Eisenhauer E. Bortezomib is active in patients with untreated or relapsed Waldenstrom’s macroglobulinemia: a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1570-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Dimopoulos MA, García-Sanz R, Gavriatopoulou M, Morel P, Kyrtsonis MC, Michalis E, Kartasis Z, Leleu X, Palladini G, Tedeschi A. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013;122:3276-3282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | O’Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 27. | Gazdic T, Svobodova E, Kubanek M, Kment M, Pagacova L, Viklicky O, Malek I, Kautzner J. Bortezomib-containing regimen for primary treatment of early antibody-mediated cardiac allograft rejection: a case report. Prog Transplant. 2015;25:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Tzvetanov I, Spaggiari M, Joseph J, Jeon H, Thielke J, Oberholzer J, Benedetti E. The use of bortezomib as a rescue treatment for acute antibody-mediated rejection: report of three cases and review of literature. Transplant Proc. 2012;44:2971-2975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Zinzani PL, Musuraca G, Tani M, Stefoni V, Marchi E, Fina M, Pellegrini C, Alinari L, Derenzini E, de Vivo A. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:4293-4297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Agathocleous A, Rohatiner A, Rule S, Hunter H, Kerr JP, Neeson SM, Matthews J, Strauss S, Montoto S, Johnson P. Weekly versus twice weekly bortezomib given in conjunction with rituximab, in patients with recurrent follicular lymphoma, mantle cell lymphoma and Waldenström macroglobulinaemia. Br J Haematol. 2010;151:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Kastritis E, Wechalekar AD, Dimopoulos MA, Merlini G, Hawkins PN, Perfetti V, Gillmore JD, Palladini G. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28:1031-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 32. | Lamm W, Willenbacher W, Lang A, Zojer N, Müldür E, Ludwig H, Schauer-Stalzer B, Zielinski CC, Drach J. Efficacy of the combination of bortezomib and dexamethasone in systemic AL amyloidosis. Ann Hematol. 2011;90:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Arnulf B, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, van de Velde H, Feng H, Cakana A, Deraedt W, Moreau P. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97:1925-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 320] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 35. | Durie BG, Hoering A, Rajkumar SV, Abidi MH, Epstein J, Kahanic SP, Thakuri MC, Reu FJ, Reynolds CM, Sexton R. Bortezomib, lenalidomide and dexamethasone vs lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell Transplant (ASCT): Results of the randomized phase III trial SWOG S0777. Blood. 2015;126:25. [Cited in This Article: ] |
| 36. | Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, Garderet L, Royer B, Brechignac S, Tiab M. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569-2574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 37. | Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, Jaye DL, Francis D, Giusti S, Torre C. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777-3784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 38. | Wu KL, Heule F, Lam K, Sonneveld P. Pleomorphic presentation of cutaneous lesions associated with the proteasome inhibitor bortezomib in patients with multiple myeloma. J Am Acad Dermatol. 2006;55:897-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:754-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1070] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 40. | San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1476] [Cited by in F6Publishing: 1409] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 41. | Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259-2266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 319] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 42. | Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, Gentili S, Patriarca F, Nozzoli C, Levi A. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745-4753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 319] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 43. | Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113-3120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 486] [Cited by in F6Publishing: 464] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 44. | Chanan-Khan A, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008;26:4784-4790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Fu H, Fang H, Sun J, Wang H, Liu A, Sun J, Wu Z. Boronic acid-based enzyme inhibitors: a review of recent progress. Curr Med Chem. 2014;21:3271-3280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Ramesh N. Takeda receives positive CHMP opinion for conditional approval for nindaro (Ixazomib) 2016. [accessed 2016 Sep 22]. Available from: http://www.myelomabeacon.com/pr/2016/09/16/ninlaro-positive-chmp-opinion-conditional-approval. [Cited in This Article: ] |
| 47. | Hedstrom L. Serine protease mechanism and specificity. Chem Rev. 2002;102:4501-4524. [PubMed] [Cited in This Article: ] |
| 48. | Murray FJ. A comparative review of the pharmacokinetics of boric acid in rodents and humans. Biol Trace Elem Res. 1998;66:331-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Fairchild RG, Kahl SB, Laster BH, Kalef-Ezra J, Popenoe EA. In vitro determination of uptake, retention, distribution, biological efficacy, and toxicity of boronated compounds for neutron capture therapy: a comparison of porphyrins with sulfhydryl boron hydrides. Cancer Res. 1990;50:4860-4865. [PubMed] [Cited in This Article: ] |
| 50. | Amorim MJ, Natal-da-Luz T, Sousa JP, Loureiro S, Becker L, Römbke J, Soares AM. Boric acid as reference substance: pros, cons and standardization. Ecotoxicology. 2012;21:919-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Wang C, Bennett GW. Cost and effectiveness of community-wide integrated pest management for German cockroach, cockroach allergen, and insecticide use reduction in low-income housing. J Econ Entomol. 2009;102:1614-1623. [PubMed] [Cited in This Article: ] |
| 52. | O’Donovan MR, Mee CD, Fenner S, Teasdale A, Phillips DH. Boronic acids-a novel class of bacterial mutagen. Mutat Res. 2011;724:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Scott H, Walmsley RM. Ames positive boronic acids are not all eukaryotic genotoxins. Mutat Res Genet Toxicol Environ Mutagen. 2015;777:68-72. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Dembitsky VM, Al Quntar AA, Srebnik M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem Rev. 2011;111:209-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Wong A, Grubb DR, Cooley N, Luo J, Woodcock EA. Regulation of autophagy in cardiomyocytes by Ins(1,4,5)P(3) and IP(3)-receptors. J Mol Cell Cardiol. 2013;54:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Del Rosso JQ, Plattner JJ. From the Test Tube to the Treatment Room: Fundamentals of Boron-containing Compounds and their Relevance to Dermatology. J Clin Aesthet Dermatol. 2014;7:13-21. [PubMed] [Cited in This Article: ] |
| 57. | About Anacor: Strategy and partnering. [accessed 2016 Nov 30]. Available from: http://www.anacor.com. [Cited in This Article: ] |
| 58. | Viñas I Teixidor C. The uniqueness of boron as a novel challenging element for drugs in pharmacology, medicine and for smart biomaterials. Future Med Chem. 2013;5:617-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Olid D, Núñez R, Viñas C, Teixidor F. Methods to produce B-C, B-P, B-N and B-S bonds in boron clusters. Chem Soc Rev. 2013;42:3318-3336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 60. | Furikado Y, Nagahata T, Okamoto T, Sugaya T, Iwatsuki S, Inamo M, Takagi HD, Odani A, Ishihara K. Universal reaction mechanism of boronic acids with diols in aqueous solution: kinetics and the basic concept of a conditional formation constant. Chemistry. 2014;20:13194-13202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc Natl Acad Sci USA. 2015;112:2401-2406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 62. | Heindel JJ, Price CJ, Schwetz BA. The developmental toxicity of boric acid in mice, rats, and rabbits. Environ Health Perspect. 1994;102 Suppl 7:107-112. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Scorei R. Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on earth. Orig Life Evol Biosph. 2012;42:3-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |