Published online Apr 12, 2013. doi: 10.5528/wjtm.v2.i1.1
Revised: January 24, 2013
Accepted: February 8, 2013
Published online: April 12, 2013
Processing time: 92 Days and 15.7 Hours
The enzyme steroid sulfatase (STS) desulfates a variety of steroid compounds thereby altering their activity. STS is expressed in the skin, and its deficiency in this tissue has been linked to the dermatological condition X-linked ichthyosis. STS is also highly expressed in the developing and adult human brain, and in a variety of steroidogenic organs (including the placenta and gonads); therefore it has the potential to influence brain development and function directly and/or indirectly (through influencing the hormonal milieu). In this review, we first discuss evidence from human and animal model studies suggesting that STS deficiency might predispose to neurobehavioural abnormalities and certain psychiatric disorders. We subsequently discuss potential mechanisms that may underlie these vulnerabilities. The data described herein have potential implications for understanding the complete spectrum of clinical phenotypes associated with X-linked ichthyosis, and may indicate novel pathogenic mechanisms underlying psychological dysfunction in developmental disorders such as attention deficit hyperactivity disorder and Turner syndrome.
- Citation: Trent S, Davies W. Cognitive, behavioural and psychiatric phenotypes associated with steroid sulfatase deficiency. World J Transl Med 2013; 2(1): 1-12
- URL: https://www.wjgnet.com/2220-6132/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v2.i1.1
Steroid sulfatase (STS, formerly known as arylsulfatase C) is an enzyme that acts as a homodimer within the endoplasmic reticulum to cleave sulfate groups from a variety of sulfated steroid hormones (notably 16α-hydroxy-dehydroepiandrosterone, dehydroepiandrosterone, estrone and cholesterol sulfates), thereby altering their biological function; the desulfation of several of these compounds represents an initial step in the biosynthesis of a number of androgens and oestrogens[1]. The STS protein is expressed in a number of tissues important in reproductive function including the placenta (highest expression), the ovaries, the testes and the mammary gland, as well as other non-reproductive tissues such as the liver and thyroid gland[2] (http://www.ncbi.nlm.nih.gov/unigene).
Besides potentially influencing the development and/or ongoing function of the aforementioned organs, STS represents an excellent a priori candidate modulator of brain development and behaviour: during human embryogenesis, STS expression occurs throughout the thalamus, in the cortical plate, throughout the basal ganglia, in the hypothalamus and anterior pituitary gland, and in the cerebellar neuroepithelium (a pattern largely consistent with that seen in other mammalian and non-mammalian species)[3], and with reported sulfatase activities in post mortem brain tissue of adult humans[4]. Given this persistent brain expression, STS is likely to influence neural function directly; the substrates and products of the enzyme are known to act as modulators at key sites of neurotransmission in the brain, notably at γ aminobutyric acid type A, N-methyl-D-aspartic acid and sigma (σ)receptors[1]. In addition, STS could exert a significant indirect influence on the brain via its role in androgen and oestrogen biosynthesis; these compounds can act systemically either to substantially and permanently alter brain development (“organisational effects’’) or to influence ongoing neural function via more subtle, potentially reversible “activational effects”[5].
In man, STS is encoded by the X-linked STS gene, which resides just outside pseudoautosomal region (PAR) 1 at Xp22.3. STS escapes X-inactivation[6], and its Y homologue is pseudogenic as a consequence of a pericentric inversion[7]. Together these attributes suggest the possibility that the gene might be expressed more highly in female than male tissues, and hence the activity of the associated enzyme may be greater in the former sex; there is some empirical evidence that this may be the case[8-10]. A recent expression analysis has suggested that alternative first exons of the STS gene may be employed in different tissues, with exons 0a and 1b being most abundant in the brain[11]. In rats, as in man, STS is X-linked, but the rat orthologue appears to be subject to X-inactivation[12]. In mice, STS is located within the PAR, and therefore by definition, escapes X-inactivation[13]. At the genetic level, there is considerable sequence divergence across species, although the function of the encoded enzyme appears to be largely conserved[12,13].
The vast majority of cases of STS deficiency (85%-90%) are caused by complete/partial deletions of the STS gene, which typically also encompass genes immediately adjacent (HDHD1A, PNPLA4 andVCX); larger, rarer deletions may encompass more distant genes including ARSE (encoding arylsulfatase E), NLGN4X (neuroligin 4X), other members of the VCX family (encoding variably charged proteins) and KAL1 (encoding anosmin-1)[14-21]. About 5%-10% of patients with STS deficiency present with a complex phenotype arising from the deletion of one or more of the disease genes listed above: altered neuroligin 4X function has previously been suggested to account for sporadic cases of autism and mental retardation[20,22,23], deficiency for the VCX and VCX3A genes has been suggested as a possible cause of mental retardation[24-26] (although there is some evidence that deletion of these genes alone is not sufficient to cause this phenotype[27-29]), arylsulfatase E dysfunction has been linked to the skeletal disorder chondrodysplasia punctata[30], and mutations within KAL1 (encoding anosmin-1) are associated with Kallman syndrome characterised by hypogonadotropic hypogonadism and anosmia[31]. The remaining 10% of cases of STS deficiency may be caused by a either variety of point mutations within the STS gene resulting in aberrant gene expression/splicing or protein function[32-39], or, rarely, by a deleterious mutation in the autosomal gene encoding sulfatase modifying factor-1 (SUMF1) whose normal role is the posttranslational modification and catalytic activation of a host of sulfatase enzymes[40]. The majority of genetic mutations at the STS locus are likely to be inherited rather than arising de novo[41].
The main phenotype associated with STS deficiency is a comparatively benign dermatological condition (X-linked ichthyosis, XLI) in which affected individuals present with large dark brown, adherent scales (particularly on the trunk, arms and legs) as a consequence of the accumulation of cholesterol sulfate in the membranes of stratum corneum cells[42]. Unsurprisingly, given that STS is X-linked, the vast majority of subjects diagnosed with XLI are male, although skin dryness may be manifest in female heterozygotes. Other, less common, phenotypes associated with STS deficiency include corneal opacities which do not affect vision (10%-50% of cases), and maldescent of the testes during embryogenesis (20% of cases)[42]. Expectant mothers carrying fetuses affected by STS deficiency tend to exhibit delayed or prolonged labour as a consequence of reduced placental oestrogen production and insufficient cervical dilation[43].
Confirmation of STS deficiency in cases ascertained clinically (generally through their skin condition) may be done biochemically through showing absent enzyme activity, or genetically through identifying either a complete/partial STS deletion or a deleterious point mutation within the gene[42]. Prevalence estimates for XLI based on clinical ascertainment have been in the range of 1 in 3000-6000 males; however, prenatal screens identifying cases of STS deficiency through low levels of maternal serum oestrogen (unconjugated estriol) have reported higher prevalences of approximately 1 in 1500 males[44,45]. These data indicate a spectrum of phenotypic consequences of enzyme deficiency, ranging from the most severe and easily ascertained (including contiguous gene syndromes), to milder forms (skin abnormalities only), to forms with no obvious clinical implications.
As the STS locus escapes X-inactivation, loss of genetic material from the short arm of the X chromosome, or loss of an entire X chromosome, in females as occurs in the developmental disorder Turner syndrome (TS)[46], will result in haploinsufficiency for STS. Whilst such haploinsufficiency is unlikely to result in phenotypes as obvious as those caused by complete enzyme deficiency, it may still feasibly contribute to the physiological and psychological abnormalities seen in TS[47]. Moreover, loss of one STS allele in TS could expose deleterious mutations on the remaining allele.
Emerging data from a variety of experimental sources is providing converging evidence for a role for STS in the modulation of attention. In the first systematic study of behaviour in individuals with XLI, Kent et al[20] showed that within a sample of 25 affected boys, ten met DSM-IV criteria for diagnosis of attention deficit hyperactivity disorder (ADHD), a neurodevelopmental condition characterised by inattention, pathological impulsivity and hyperactivity[48]; crucially, this sample was originally ascertained on the basis of low unconjugated estriol levels in their pregnant mothers and not on the basis of the boys’ behaviour. Of the ten individuals affected by ADHD, eight were diagnosed with primarily inattentive subtype of the disorder, whilst the remaining two were diagnosed with the combined subtype (exhibiting evidence of inattention, and impulsivity and/or hyperactivity). Although this study did not employ a matched-control group, the 40% overall ADHD diagnosis rate (and 32% inattentive ADHD diagnosis rate) within the XLI group was substantially higher than that typically observed within the United Kingdom general population (4%-6% overall, 0.5% inattentive). Importantly, whilst most boys diagnosed with inattentive ADHD had deletions spanning multiple genes, two individuals within this subgroup had presumed inactivating point mutations within STS, indicating that STS dysfunction per se might predispose to inattention rather than the lack of gene product from a contiguous gene. The findings of the Kent et al[20] study are consistent with previous, more limited, case reports in the literature that have described individuals with contiguous Xp22.3 gene deletions and ADHD[49-51]. These initial data indicate that genetic screening of large, behaviourally-ascertained ADHD samples and appropriate control samples to investigate the relative prevalence of STS deletions/point mutations may be worthwhile.
Whilst work stimulated by the initial XLI findings has indicated no significant association between polymorphisms within STS and overall ADHD risk after correction for multiple testing[3,11], there does appear to be a significant, and replicable, association between variation at rs17268988 (located within intron 9 of STS) and number of inattentive symptoms within ADHD cohorts[3,52]; specifically, possession of the minor G allele at this site (allele frequency about 0.25) is associated with a greater number of inattentive symptoms, particularly in older children (> 9 years of age). Whilst the genetic, cellular and neural mechanisms through which this association is mediated remain obscure, this finding provides further evidence for a role of STS in attentional function in neurodevelopmentally-compromised subjects; the extent to which an association between this genetic variant and attention exists in healthy individuals remains to be tested. Other polymorphisms across the STS gene (rs12861247, rs5978405 and rs5933863) have been shown to be significantly associated with aspects of cognitive function in males with ADHD as indexed by their performance on the comprehension, verbal IQ and picture completion Wechsler subtests, respectively[3]; as a small sample size was employed in this study these findings could be spurious, but if confirmed, these associations could feasibly also be mediated via effects on attention.
One of the most consistently reported neuropsychological findings in women with TS is inattention[53] which can be manifest as heightened distractability in real-life situations[54]. Rates of ADHD have been reported to be up to eighteen-fold higher in the TS population than in a control female population[55]. By correlating individual TS subjects’ aggregate cognitive scores (partly based upon measures of attention) with their karyotype, Zinn et al[56] concluded that haploinsufficiency for an 8.3Mb region of chromosome Xp22.3 housing just 31 annotated genes (including STS) was critical in the development of the characteristic TS cognitive profile. Given the results arising from the XLI and ADHD studies described above, STS is a candidate for the attentional component of this profile. As such, it will be interesting to test whether those subjects with TS at greatest risk of attention deficits are hemizygous for the previously-identified risk alleles or deleterious mutations within STS. Should this prove to be the case, it would offer an opportunity to provide better genetic counselling and earlier clinical intervention in cases of TS.
Attention deficits are a prominent clinical feature of neuropsychiatric disorders other than ADHD, including autism[57-59] and schizophrenia[60]; cytogenetic deletions encompassing STS have been reported in individuals affected by both disorders[20,61-65]. Psychiatric disorders associated with attention problems are more common (e.g., ADHD and autism[66]) or more severe (e.g., schizophrenia[67,68]) in males than in females. Thus, it is plausible that the lower expression/activity of STS in males reduces their threshold of vulnerability to attentional dysfunction.
Recent data from animal model work appears to substantiate the link between STS dysfunction and inattention. Performance deficits in the 39, XO mouse (a model of TS[69]) on the 5-choice serial reaction time task (5-CSRTT) assaying visuospatial attention could be rescued by the addition of a small chromosome containing a small number of additional genes including STS[70]. Subsequent work in another genetic model, the 39, XY*O mouse (in which the STS gene is deleted as a consequence of an end-to-end fusion of the X and Y chromosomes within the PAR), revealed that these mice are less able to detect stimuli of short duration than wildtype mice[71]. Parallel studies in mice in which the STS axis was acutely pharmacologically modulated also showed effects on attention; specifically, administration of the enzyme substrate dehydroepiandrosterone sulfate (DHEAS) enhanced a main index of attention, whilst administration of the specific enzyme inhibitor COUMATE impaired response accuracy under attentionally-demanding conditions[71]. These pharmacological data, besides hinting that brain DHEA(S) levels may be a pertinent factor in attentional function, also indicate that ongoing STS activity could influence this psychological process. Given that peripherally-administered DHEAS is rapidly converted to DHEA within the mammalian brain[72], it is plausible that high levels of the latter compound within the brain are associated with enhanced attention, but that low levels (as presumably occur in XLI patients and 39, XY*O mice) are associated with impaired attention. Whilst animal model work has provided some preliminary clues as to brain pathways that might be affected by STS and hence which might underpin its effect on attention (see later), more in-depth analyses are clearly required. As STS appears to influence ongoing attentional processes, such analyses may feasibly identify novel therapeutic targets that could be acutely pharmacologically modulated in adolescents and adults affected by disorders of attention.
Early genetic evidence in mice examining inter-male aggression indicated that the Y chromosome PAR played an important role[73]; in mice, the PAR was originally thought to house just one gene (STS), but recently a second mouse PAR gene Asmt (encoding the enzyme acetylserotonin O-methyltransferase involved in the biosynthesis of melatonin from serotonin) has been identified[74]. However, in support of STS as a candidate mediator of this phenotype, a strong relationship between protein levels and aggression has been noted across several inbred mouse strains[75] and co-administration of both DHEAS and COUMATE resulted in heightened aggression in the inbred CBA/H strain[76]; this latter result suggests that, in addition to modulating ongoing attentional function in mice, STS may also modulate ongoing levels of aggressive behaviour. Consistent with the results of this pharmacological study, 39, XY*O male mice, which have low levels of DHEA (and presumably elevated levels of DHEAS), are hyper-aggressive towards their cagemates[77].
To date, there is little evidence from human studies that suggests an equivalent role for STS in modulating aggression: to our knowledge, abnormally high levels of overt aggression have not been reported in cases of STS deletion, and within our Cardiff ADHD sample we did not detect association between a number of polymorphisms within STS and DSM-IV aggressive symptoms (albeit the case that the sample displayed low overall levels of aggression)[3]. There are two possible reasons for this apparent cross-species discrepancy: first, mouse brain function may be differentially affected by changes in STS levels, or the social structures imposed within groups of laboratory-housed mice might be conducive to elicting aggression. Alternatively, the phenotype of elevated aggression may be present in STS-deficient humans but it may be more subtle than in mice, or only observable upon provocation.
Whilst there is fairly convincing cross-species evidence indicating a role for STS in attentional processes, and some data consistent with a role for the enzyme in aggression (in rodents at least), data suggesting a modulatory role in other behavioural phenotypes is more limited; this preliminary evidence is summarised below.
In addition to being inattentive relative to 40, XY control mice, STS-deficient 39, XY*O mice appear to be less impulsive, as indexed by their tendency to make fewer premature responses to a stimulus in the 5-CSRTT[71], and to better withhold responding on a murine analogue of the human Stop Signal Reaction Time Task (SSRT); pharmacological manipulation of the STS axis (i.e., COUMATE and DHEAS administration) also enhanced behavioural inhibition on the SSRT. These data hint that STS deficiency in humans may also confer reduced impulsivity. Most research in psychiatry has focussed on pathological hyper-impulsiveness rather than the consequences of hypo-impulsivity, so exactly how this might be manifest is unclear - perhaps such individuals would show a tendency towards apathy or extreme risk aversion To date, there is no information available regarding whether XLI patients are particularly apathetic or cautious. At the neuropsychological level, we might predict that STS-deficient subjects, like 39, XY*O mice, would demonstrate enhanced “stopping” on the SSRT. Testing for association between polymorphisms across the STS gene and DSM-IV impulsive symptoms in a small sample of boys with ADHD revealed no significant findings[3]. However, such a result is perhaps not surprising if STS variation does generally result in reduced impulsivity, given that the ADHD population is, by definition, ascertained on the basis of abnormally high levels of impulsivity.
Postpartum psychosis (PP) is a severe psychiatric condition occurring shortly after birth in 1-2 of every 1000 new mothers; it is characterised by hallucinations/delusions, cognitive disorganisation, mood changes and sleep abnormalities and can occasionally result in self- or infant-directed harm[78]. Whilst biology undoubtedly plays a role in PP susceptibility, as yet, well-defined risk factors are few and far between. The biggest risk factor appears to be a personal or family history for psychiatric (psychotic) illness, with a strong and consistent relationship between prior bipolar disorder diagnosis and vulnerability to PP; other risk factors include the extent to which circulating maternal oestrogen levels plummet following expulsion of the placenta, and levels of maternal stress[78]. It has been proposed that maternal STS deficiency might influence PP risk on the basis of several observations (described in detail in a recent paper[79]): (1) levels of the STS enzyme in the mammalian maternal brain specifically increase following parturition, hence perturbation of the STS axis as a consequence of enzyme deficiency may particularly impact upon behaviour at this timepoint; (2) the enzyme is highly expressed in the placenta where it is involved in the biosynthesis of oestrogen precursors; decreased levels of circulating oestrogens during pregnancy and in the postpartum period as a consequence of Sts deficiency may predispose to psychosis; (3) STS is a candidate gene underlying a quantitative trait locus for postpartum behavioural disturbance in pigs; (4) as discussed above, STS-deficiency in mice and humans predisposes to inattention (or “cognitive disorganisation”) and occasional aggression; and (5) STS is highly expressed in the thyroid gland, an organ whose dysfunction is often noted in cases of PP. Given the overlap between bipolar disorder and PP, it is interesting to note that in a recent meta-analysis of gene expression data from post mortem brain samples from patients with bipolar disorder, STS was one of just a handful of genes whose expression was consistently downregulated[80]; thus it plausible that STS deficiency predisposes primarily to bipolar disorder and thereafter to PP, or to PP directly, or to both disorders via shared neurobiological mechanisms. It is also noteworthy that the estimated prevalence of female heterozygosity for a deleterious STS mutation (based on prenatal screening and XLI rates) is comparable to that of PP (i.e., about 1 in 750-3000 females), that the neurosteroid system has previously been implicated in psychotic disorders and in other postpartum conditions[81], that two female cases of paranoid schizophrenia possessed cytogenetic deletions spanning STS[61], and that deficiency for the STS paralogue, ARSA (arylsulfatase A), has been linked to psychotic phenotypes and postpartum depression[82-84].
A relationship between STS deficiency and a second mood disorder, unipolar depression, might also be considered, although currently the evidence for such a link is weak or anecdotal. One of two females completely deficient for enzyme activity according to biochemical measurements (and therefore homozygous for an undefined STS mutation) was reported to have a history of depression[85]. Moreover, we are aware of one United Kingdom family in which X-linked ichthyosis appears to co-segregate with debilitating depression/anxiety.
39, XY*O mice are hyperactive, particularly within a novel environment and during the night (i.e., during their active phase)[77], and there is a significant inverse relationship between evening activity in a novel environment and systemic levels of the STS product DHEA[86]. 39, XY*O mice also exhibit increased response rates within operant behavioural tests where only one type of response is required, which may reflect a greater tendency towards perseverative responding[86,87]. Finally, these mice tend to show heightened emotional reactivity relative to wildtype controls as indexed by their lack of willingness to enter aversive (open, brightly-lit) spaces, and their increased levels of urination and defecation in such spaces[77]. As 39, XY*O mice also lack the PAR gene Asmt[86], theoretically, the hyperactivity, perseverative and emotional reactivity phenotypes in 39, XY*O mice could be due to the loss of STS and/or Asmt (particularly given that the former and latter phenotypes are not elicited upon acute COUMATE administration[77]). However, the inverse association between DHEA levels and hyperactivity tends to argue for a specific role for STS in this behaviour. To date, it has not proved possible to generate single-gene STS and Asmt knockout rodents; should such animals be created in the future, they could be used to dissociate between behavioural effects arising dues to STS and/or ASMT deficiency.
Hyperactivity and behavioural inflexibility (perseveration) have not consistently been reported as (endo) phenotypes associated with cases of STS deficiency in humans. This could be because these phenotypes in mice are largely due to ASMT deficiency, because there is no association, because the phenotypes are relatively minor and do not impair everyday function, or because no systematic, objective, case-controlled behavioural studies have yet been performed for XLI (e.g., using activity monitors). The observation that 39, XY*O mice exhibit more anxiety-related behaviours than their wildtype counterparts is consistent with anecdotal evidence suggesting anxiety in XLI patients, but clearly the veracity and magnitude of this association requires further exploration.
The comparative rarity of XLI patients, together with the relative inaccessibility of the human brain, means that, to date, little is known about the neuroanatomical and neurochemical sequelae of STS deficiency in man. Individuals in which STS is deleted have been reported to exhibit cortical malformations including polymicrogyria[88] and heterotopia[89,90]; whilst these manifestations may be consistent with a role for STS in the developing cortex[3], given that these individuals lack multiple genes at Xp22.3, these abnormalities might equally likely be a consequence of the absence of function of one or more contiguous brain-expressed genes (e.g., HDHD1A). To uniquely ascribe these cortical phenotypes to STS deficiency, it will be necessary to examine the brains of individuals with nonsense point mutations within STS either by in vivo neuroimaging or through analysing post mortem tissue.
Rodent models are far more amenable to neurobiological investigation than humans, and ongoing studies in genetic and pharmacological models have provided interesting initial clues as to the neurochemical mechanisms underlying STS deficiency effects on behaviour. Relatively crude analyses of whole tissue monoamine levels in the 39, XY*O mouse have identified brain region-specific changes in the serotonin (5-HT) system (notably elevated 5-HT levels in the striatum and hippocampus)[86,87]; these mutant mice also have increased hippocampal expression of the Htr2c gene (encoding the 5-HT2c receptor) and reduced striatal levels of the noradrenaline metabolite 4-hydroxy-3-methoxyphenylglycol[87]. Correlational analyses have indicated a positive linear relationship between hippocampal 5-HT levels and response rate/behavioural perseveration across two independent behavioural paradigms[86,87] and between striatal 5-HT levels and activity[87]; whilst these observations suggest that the 5-HT system abnormalities may affect these behavioural endophenotypes, an explicit causal link between the variables has yet to be established. Interestingly, serotonergic system dysfunction (including disruption of 5-HT2c receptor expression/function) has been implicated in many of the psychiatric phenotypes linked to STS deficiency including inattention[91,92], aggression[93], impulsivity[94], PP[95], anxiety and depression[96,97]. More refined analyses of the relationship between analogues of these behavioural/psychiatric outcomes and 5-HT perturbation in the 39, XY*O mouse will be useful, notably investigating whether neurochemical changes within specific sub-regions of the hippocampus and striatum underlie the behavioural abnormalities. Although the most parsimonious explanation for the neurochemical findings is STS deficiency[86], it is formally possible that they could arise due as a consequence of Asmt gene deletion. Again, examining single gene knockouts and/or assaying whether 39, XY*O phenotypes are recapitulated by acute enzyme inhibition will aid in distinguishing between these scenarios. Should a discrete STS-dependent change in 5-HT function be confirmed in mice, its potential functional relevance to human phenotypes might be tested through using positron emission tomography with serotonergic ligands in XLI patients[98].
To date, the neuroanatomy of the 39, XY*O mouse has not been examined; future analyses might initially look for gross abnormalities in hippocampal and striatal morphology for example, before investigating more subtle changes in cell number or subtype in these regions. Given the suggestion above that XLI may be associated with cortical abnormalities, examining the development and structure of the cortex in STS-deficient mice may also be warranted.
Rat studies in which STS is inhibited have also begun to shed some light on the neurochemical pathways influenced by enzyme dysfunction. Again, these have emphasised the hippocampus, a key site of neurosteroid-mediated neurogenesis[99], as an important locus of ongoing Sts action. Initial studies using estrone-3-O-sulfamate (EMATE) as an inhibitor indicated that inducing acute enzyme dysfunction, particularly in combination with substrate (DHEAS) administration, could benefit learning and/or memory formation[100]. However, EMATE is oestrogenic[101], and thus it is difficult to ascertain whether its effects on cognition are due to its inhibitory and/or its oestrogenic role. In later studies, systemic enzyme inhibition using the compound [p-O-sulfamoyl-N-tetradecanoyl tyramine (DU-14), a compound with lower oestrogenicity than EMATE] administered chronically was shown to increase brain levels of DHEAS[102], enhance hippocampal release of the neurotransmitter acetylcholine[103], and result in improved learning, spatial memory and context-dependent fear memory[104,105]. The acetylcholine system has a long association with attentional function, and one obvious route through which STS axis variation might influence attention is via this intermediary mechanism[106]. Thus, future work might examine the effects of STS inhibition, or STS gene deletion, on acetylcholinergic function in the hippocampus and other brain regions implicated in attention, and how these induced neurochemical changes might then relate to measures of (in) attention. Within the hippocampus, various serotonergic receptors are involved in controlling acetylcholine release[107]; hence, it will also be interesting to see whether there is any relationship between serotonergic and acetylcholinergic abnormalities induced as a consequence of STS deficiency in this structure.
In the preceding text we have marshalled evidence from a variety of sources which indicates that STS deficiency may elicit a multitude of brain and behavioural phenotypes of relevance to psychiatric vulnerability. Clearly there is a need for further systematic work to specify the precise neural, behavioural and psychiatric endophenotypes arising from this molecular abnormality, and to investigate their prevalence; such work may provide more general insights into behavioural and cognitive processes that commonly go awry in psychiatric conditions (e.g., attention). Ideally, this work would involve the examination of individuals with discrete mis-/nonsense point mutations within the STS gene, or deletions solely encompassing STS, in whom any phenotype could not be ascribed to missing contiguous genes; however, given the low frequency of such mutations within the population, identifying such individuals will be difficult, and establishing reliable prevalence figures for particular phenotypes will be challenging. In light of the arguments outlined above, we propose that it would be worthwhile for patients with confirmed/suspected XLI to be asked by clinicians about any behavioural problems that they might have experienced, given that any link between dermatologic abnormalities and such difficulties may not be intuitive.
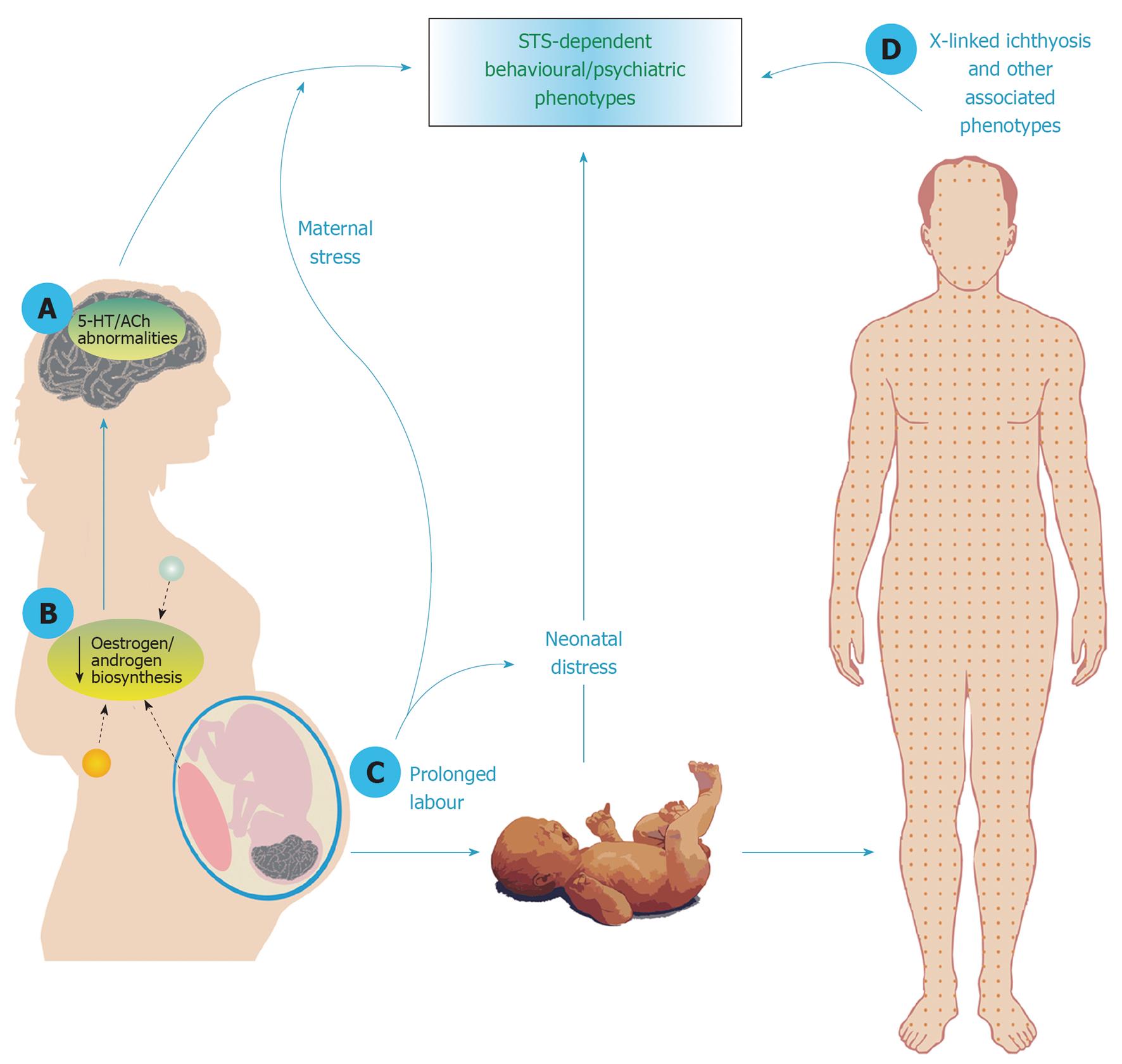
Should elevated rates of psychiatric illness be identified in enzyme-deficient individuals, it will be important to characterise their mechanistic basis i.e., whether they are related to loss-of-function of the enzyme in the brain per se, to the pleiotropic, non-brain effects of protein dysfunction, or to both (Figure 1). STS deficiency could influence brain function and behaviour directly (e.g., through modulating the serotonergic or acetylcholinergic systems). Alternatively, lack of enzyme expression in steroidogenic organs such as the placenta, gonads and mammary gland[108] could result in reduced levels of circulating steroid hormones (notably androgens and oestrogens) and subsequent downstream organisational and/or activational effects on brain development and function. Animals models in which STS function is perturbed will be of use in distinguishing between these two possibilities. Furthermore, it is conceivable that an increased risk of some psychiatric disorders (e.g., depression and anxiety) could result from individuals having to live with potentially disfiguring somatic conditions associated with enzyme deficiency including ichthyosis[42], hypogenitalism[109], or male-pattern baldness[110]; in support of this notion, one study has shown that patients with ichthyosis have a lower health-related quality of life[111]. Finally, it is conceivable that STS deficiency in the offspring could both result in increased risk of psychiatric illness in that individual simply as a consequence of neonatal distress arising from prolonged maternal labour[112-114], and, by the same general stress-inducing mechanism, could increase risk of postpartum mental illness in the mother[115]. To determine whether these latter mechanisms may be important, comparison of rates of behavioural/psychiatric abnormalities in STS-deficient cases with those in subjects with other similar skin conditions for example, or exposed to other causes of prolonged labour, may be valuable.
An alternative strategy for determining the consequences of acutely impaired STS function may be to explicitly test for brain and behavioural alterations in subjects administered enzyme inhibitors. 667-COUMATE (Irosustat) has been proposed as a treatment for hormone-dependent cancers[116,117] and for endometriosis[118], conditions where biosynthesis of oestrogens and androgens must be restricted. Although no obvious psychological side-effects of 667-COUMATE treatment were reported in the first clinical trial of the drug in breast cancer patients[119], the animal data discussed above suggest that subtle effects on cognition (attention, impulsivity) and behaviour (aggression) might be anticipated. In assessing whether this is the case, potential confounding variables such as baseline rates of depression, age, or the potential behavioural effects of co-administered therapeutic drugs, in patients would have to be considered.
Finally, taking a conceptually different approach, it will be interesting to see whether subjects with cytogenetic duplications encompassing STS exhibit any clear brain or behavioural phenotypes. However, in most, if not all, of these cases, any data will be confounded by duplication of contiguous brain-expressed genes. To date, the small number of cases with Xp22.3 duplications reported in the literature either do not appear to exhibit any severe neuropsychological phenotypes[120], or present with relatively non-specific phenotypes such as learning disability and/or developmental delay[121] depending upon the size of the duplication.
Understanding if, and how, STS deficiency influences vulnerability to psychiatric illness will be important in terms of counselling for XLI (and potentially TS), and additionally may highlight novel therapeutically-amenable targets for common aspects of psychological dysfunction.
Core tip: The enzyme steroid sulfatase (STS) cleaves sulfate groups from neuroactive steroid hormones thereby altering their activity. Here, we review cross-species evidence indicating that deficiency for this enzyme might influence behaviour and vulnerability to psychiatric illness; we then suggest potential mediating mechanisms. Understanding whether or not STS deficiency impacts upon neural function, and if so, how, has potential implications for diagnosis, counselling and treatment in cases of X-linked ichthyosis (the dermatological condition associated with STS deficiency). Moreover, this understanding may provide more general novel insights into the pathogenesis of common and disabling psychiatric disorders.
P- Reviewers Cuevas-Covarrubias SA, Kent L S- Editor Zhai HH L- Editor A E- Editor Zheng XM
| 1. | Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005;26:171-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 388] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 2. | Selcer KW, Difrancesca HM, Chandra AB, Li PK. Immunohistochemical analysis of steroid sulfatase in human tissues. J Steroid Biochem Mol Biol. 2007;105:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Stergiakouli E, Langley K, Williams H, Walters J, Williams NM, Suren S, Giegling I, Wilkinson LS, Owen MJ, O’Donovan MC. Steroid sulfatase is a potential modifier of cognition in attention deficit hyperactivity disorder. Genes Brain Behav. 2011;10:334-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Perumal AS, Robins E. Regional and subcellular distribution of aryl-and steroid sulfatases in brain. Brain Res. 1973;59:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 2011;32:183-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Mohandas T, Sparkes RS, Hellkuhl B, Grzeschik KH, Shapiro LJ. Expression of an X-linked gene from an inactive human X chromosome in mouse-human hybrid cells: further evidence for the noninactivation of the steroid sulfatase locus in man. Proc Natl Acad Sci USA. 1980;77:6759-6763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Yen PH, Marsh B, Allen E, Tsai SP, Ellison J, Connolly L, Neiswanger K, Shapiro LJ. The human X-linked steroid sulfatase gene and a Y-encoded pseudogene: evidence for an inversion of the Y chromosome during primate evolution. Cell. 1988;55:1123-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Migeon BR, Shapiro LJ, Norum RA, Mohandas T, Axelman J, Dabora RL. Differential expression of steroid sulphatase locus on active and inactive human X chromosome. Nature. 1982;299:838-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Cuevas-Covarrubias SA, Juárez-Oropeza MA, Miranda-Zamora R, Díaz-Zagoya JC. Comparative analysis of human steroid sulfatase activity in prepubertal and postpubertal males and females. Biochem Mol Biol Int. 1993;30:691-695. [PubMed] |
| 10. | Steckelbroeck S, Nassen A, Ugele B, Ludwig M, Watzka M, Reissinger A, Clusmann H, Lütjohann D, Siekmann L, Klingmüller D. Steroid sulfatase (STS) expression in the human temporal lobe: enzyme activity, mRNA expression and immunohistochemistry study. J Neurochem. 2004;89:403-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Brookes KJ, Hawi Z, Park J, Scott S, Gill M, Kent L. Polymorphisms of the steroid sulfatase (STS) gene are associated with attention deficit hyperactivity disorder and influence brain tissue mRNA expression. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Li XM, Salido EC, Gong Y, Kitada K, Serikawa T, Yen PH, Shapiro LJ. Cloning of the rat steroid sulfatase gene (Sts), a non-pseudoautosomal X-linked gene that undergoes X inactivation. Mamm Genome. 1996;7:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Salido EC, Li XM, Yen PH, Martin N, Mohandas TK, Shapiro LJ. Cloning and expression of the mouse pseudoautosomal steroid sulphatase gene (Sts). Nat Genet. 1996;13:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Bonifas JM, Morley BJ, Oakey RE, Kan YW, Epstein EH. Cloning of a cDNA for steroid sulfatase: frequent occurrence of gene deletions in patients with recessive X chromosome-linked ichthyosis. Proc Natl Acad Sci USA. 1987;84:9248-9251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Gillard EF, Affara NA, Yates JR, Goudie DR, Lambert J, Aitken DA, Ferguson-Smith MA. Deletion of a DNA sequence in eight of nine families with X-linked ichthyosis (steroid sulphatase deficiency). Nucleic Acids Res. 1987;15:3977-3985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Shapiro LJ, Yen P, Pomerantz D, Martin E, Rolewic L, Mohandas T. Molecular studies of deletions at the human steroid sulfatase locus. Proc Natl Acad Sci USA. 1989;86:8477-8481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Valdes-Flores M, Kofman-Alfaro SH, Vaca AL, Cuevas-Covarrubias SA. Mutation report: a novel partial deletion of exons 2-10 of the STS gene in recessive X-linked ichthyosis. J Invest Dermatol. 2000;114:591-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Valdes-Flores M, Kofman-Alfaro SH, Vaca AL, Cuevas-Covarrubias SA. Deletion of exons 1-5 of the STS gene causing X-linked ichthyosis. J Invest Dermatol. 2001;116:456-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Jimenez Vaca AL, Valdes-Flores Mdel R, Rivera-Vega MR, González-Huerta LM, Kofman-Alfaro SH, Cuevas-Covarrubias SA. Deletion pattern of the STS gene in X-linked ichthyosis in a Mexican population. Mol Med. 2001;7:845-849. [PubMed] |
| 20. | Kent L, Emerton J, Bhadravathi V, Weisblatt E, Pasco G, Willatt LR, McMahon R, Yates JR. X-linked ichthyosis (steroid sulfatase deficiency) is associated with increased risk of attention deficit hyperactivity disorder, autism and social communication deficits. J Med Genet. 2008;45:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Cañueto J, Ciria S, Hernández-Martín A, Unamuno P, González-Sarmiento R. Analysis of the STS gene in 40 patients with recessive X-linked ichthyosis: a high frequency of partial deletions in a Spanish population. J Eur Acad Dermatol Venereol. 2010;24:1226-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 548] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 23. | Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008;16:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Fukami M, Kirsch S, Schiller S, Richter A, Benes V, Franco B, Muroya K, Rao E, Merker S, Niesler B. A member of a gene family on Xp22.3, VCX-A, is deleted in patients with X-linked nonspecific mental retardation. Am J Hum Genet. 2000;67:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Van Esch H, Hollanders K, Badisco L, Melotte C, Van Hummelen P, Vermeesch JR, Devriendt K, Fryns JP, Marynen P, Froyen G. Deletion of VCX-A due to NAHR plays a major role in the occurrence of mental retardation in patients with X-linked ichthyosis. Hum Mol Genet. 2005;14:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Hosomi N, Oiso N, Fukai K, Hanada K, Fujita H, Ishii M. Deletion of distal promoter of VCXA in a patient with X-linked ichthyosis associated with borderline mental retardation. J Dermatol Sci. 2007;45:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Cuevas-Covarrubias SA, González-Huerta LM. Analysis of the VCX3A, VCX2 and VCX3B genes shows that VCX3A gene deletion is not sufficient to result in mental retardation in X-linked ichthyosis. Br J Dermatol. 2008;158:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Macarov M, Zeigler M, Newman JP, Strich D, Sury V, Tennenbaum A, Meiner V. Deletions of VCX-A and NLGN4: a variable phenotype including normal intellect. J Intellect Disabil Res. 2007;51:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Mochel F, Missirian C, Reynaud R, Moncla A. Normal intelligence and social interactions in a male patient despite the deletion of NLGN4X and the VCX genes. Eur J Med Genet. 2008;51:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Franco B, Meroni G, Parenti G, Levilliers J, Bernard L, Gebbia M, Cox L, Maroteaux P, Sheffield L, Rappold GA. A cluster of sulfatase genes on Xp22.3: mutations in chondrodysplasia punctata (CDPX) and implications for warfarin embryopathy. Cell. 1995;81:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 201] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Cadman SM, Kim SH, Hu Y, González-Martínez D, Bouloux PM. Molecular pathogenesis of Kallmann’s syndrome. Horm Res. 2007;67:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Basler E, Grompe M, Parenti G, Yates J, Ballabio A. Identification of point mutations in the steroid sulfatase gene of three patients with X-linked ichthyosis. Am J Hum Genet. 1992;50:483-491. [PubMed] |
| 33. | Alperin ES, Shapiro LJ. Characterization of point mutations in patients with X-linked ichthyosis. Effects on the structure and function of the steroid sulfatase protein. J Biol Chem. 1997;272:20756-20763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Morita E, Katoh O, Shinoda S, Hiragun T, Tanaka T, Kameyoshi Y, Yamamoto S. A novel point mutation in the steroid sulfatase gene in X-linked ichthyosis. J Invest Dermatol. 1997;109:244-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Oyama N, Satoh M, Iwatsuki K, Kaneko F. Novel point mutations in the steroid sulfatase gene in patients with X-linked ichthyosis: transfection analysis using the mutated genes. J Invest Dermatol. 2000;114:1195-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | González-Huerta LM, Riviera-Vega MR, Kofman-Alfeuro SH, Cuevas-Covarrubias SA. Novel missense mutation (Arg432Cys) in a patient with steroid sulphatase-deficiency. Clin Endocrinol (Oxf). 2003;59:263-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Ghosh D. Mutations in X-linked ichthyosis disrupt the active site structure of estrone/DHEA sulfatase. Biochim Biophys Acta. 2004;1739:1-4. [PubMed] |
| 38. | Matsumoto J, Ariyoshi N, Ishii I, Kitada M. Six novel single nucleotide polymorphisms of the steroid sulfatase gene in a Japanese population. Drug Metab Pharmacokinet. 2010;25:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Winge MC, Hoppe T, Liedén A, Nordenskjöld M, Vahlquist A, Wahlgren CF, Törmä H, Bradley M, Berne B. Novel point mutation in the STS gene in a patient with X-linked recessive ichthyosis. J Dermatol Sci. 2011;63:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Diez-Roux G, Ballabio A. Sulfatases and human disease. Annu Rev Genomics Hum Genet. 2005;6:355-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Cuevas-Covarrubias SA, Valdes-Flores M, Orozco Orozco E, Díaz-Zagoya JC, Kofman-Alfaro SH. Most “sporadic” cases of X-linked ichthyosis are not de novo mutations. Acta Derm Venereol. 1999;79:143-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Bradshaw KD, Carr BR. Placental sulfatase deficiency: maternal and fetal expression of steroid sulfatase deficiency and X-linked ichthyosis. Obstet Gynecol Surv. 1986;41:401-413. [PubMed] |
| 44. | Langlois S, Armstrong L, Gall K, Hulait G, Livingston J, Nelson T, Power P, Pugash D, Siciliano D, Steinraths M. Steroid sulfatase deficiency and contiguous gene deletion syndrome amongst pregnant patients with low serum unconjugated estriols. Prenat Diagn. 2009;29:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Craig WY, Roberson M, Palomaki GE, Shackleton CH, Marcos J, Haddow JE. Prevalence of steroid sulfatase deficiency in California according to race and ethnicity. Prenat Diagn. 2010;30:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Bondy CA, Cheng C. Monosomy for the X chromosome. Chromosome Res. 2009;17:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Hong DS, Reiss AL. Cognition and behavior in Turner syndrome: a brief review. Pediatr Endocrinol Rev. 2012;9 Suppl 2:710-712. [PubMed] |
| 48. | Thapar A, Cooper M, Jefferies R, Stergiakouli E. What causes attention deficit hyperactivity disorder. Arch Dis Child. 2012;97:260-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 49. | Tobias ES, Bryce G, Farmer G, Barton J, Colgan J, Morrison N, Cooke A, Tolmie JL. Absence of learning difficulties in a hyperactive boy with a terminal Xp deletion encompassing the MRX49 locus. J Med Genet. 2001;38:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Boycott KM, Parslow MI, Ross JL, Miller IP, Bech-Hansen NT, MacLeod PM. A familial contiguous gene deletion syndrome at Xp22.3 characterized by severe learning disabilities and ADHD. Am J Med Genet A. 2003;122A:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Lonardo F, Parenti G, Luquetti DV, Annunziata I, Della Monica M, Perone L, De Gregori M, Zuffardi O, Brunetti-Pierri N, Andria G. Contiguous gene syndrome due to an interstitial deletion in Xp22.3 in a boy with ichthyosis, chondrodysplasia punctata, mental retardation and ADHD. Eur J Med Genet. 2007;50:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Brookes KJ, Hawi Z, Kirley A, Barry E, Gill M, Kent L. Association of the steroid sulfatase (STS) gene with attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1531-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Knickmeyer RC. Turner syndrome: advances in understanding altered cognition, brain structure and function. Curr Opin Neurol. 2012;25:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Rovet J, Ireland L. Behavioral phenotype in children with Turner syndrome. J Pediatr Psychol. 1994;19:779-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Russell HF, Wallis D, Mazzocco MM, Moshang T, Zackai E, Zinn AR, Ross JL, Muenke M. Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. J Pediatr Psychol. 2006;31:945-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Zinn AR, Roeltgen D, Stefanatos G, Ramos P, Elder FF, Kushner H, Kowal K, Ross JL. A Turner syndrome neurocognitive phenotype maps to Xp22.3. Behav Brain Funct. 2007;3:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. J Autism Dev Disord. 2006;36:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Lee DO, Ousley OY. Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome. J Atten Disord. 2009;13:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1912] [Cited by in RCA: 1854] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 61. | Milunsky J, Huang XL, Wyandt HE, Milunsky A. Schizophrenia susceptibility gene locus at Xp22.3. Clin Genet. 1999;55:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Thomas NS, Sharp AJ, Browne CE, Skuse D, Hardie C, Dennis NR. Xp deletions associated with autism in three females. Hum Genet. 1999;104:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Chocholska S, Rossier E, Barbi G, Kehrer-Sawatzki H. Molecular cytogenetic analysis of a familial interstitial deletion Xp22.2-22.3 with a highly variable phenotype in female carriers. Am J Med Genet A. 2006;140:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Vorstman JA, Staal WG, van Daalen E, van Engeland H, Hochstenbach PF, Franke L. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry. 2006;11:1, 18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 65. | Shinawi M, Patel A, Panichkul P, Zascavage R, Peters SU, Scaglia F. The Xp contiguous deletion syndrome and autism. Am J Med Genet A. 2009;149A:1138-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry. 2003;44:1092-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 447] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 67. | Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 499] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 68. | Zhang XY, Chen da C, Xiu MH, Yang FD, Haile CN, Kosten TA, Kosten TR. Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients. J Clin Psychiatry. 2012;73:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Lynn PM, Davies W. The 39,XO mouse as a model for the neurobiology of Turner syndrome and sex-biased neuropsychiatric disorders. Behav Brain Res. 2007;179:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Davies W, Humby T, Isles AR, Burgoyne PS, Wilkinson LS. X-monosomy effects on visuospatial attention in mice: a candidate gene and implications for Turner syndrome and attention deficit hyperactivity disorder. Biol Psychiatry. 2007;61:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Davies W, Humby T, Kong W, Otter T, Burgoyne PS, Wilkinson LS. Converging pharmacological and genetic evidence indicates a role for steroid sulfatase in attention. Biol Psychiatry. 2009;66:360-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Nicolas LB, Fry JP. The steroid sulfatase inhibitor COUMATE attenuates rather than enhances access of dehydroepiandrosterone sulfate to the brain in the mouse. Brain Res. 2007;1174:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Roubertoux PL, Carlier M, Degrelle H, Haas-Dupertuis MC, Phillips J, Moutier R. Co-segregation of intermale aggression with the pseudoautosomal region of the Y chromosome in mice. Genetics. 1994;136:225-230. [PubMed] |
| 74. | Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci USA. 2010;107:6412-6417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 75. | Le Roy I, Mortaud S, Tordjman S, Donsez-Darcel E, Carlier M, Degrelle H, Roubertoux PL. Genetic correlation between steroid sulfatase concentration and initiation of attack behavior in mice. Behav Genet. 1999;29:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Nicolas LB, Pinoteau W, Papot S, Routier S, Guillaumet G, Mortaud S. Aggressive behavior induced by the steroid sulfatase inhibitor COUMATE and by DHEAS in CBA/H mice. Brain Res. 2001;922:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Trent S, Dennehy A, Richardson H, Ojarikre OA, Burgoyne PS, Humby T, Davies W. Steroid sulfatase-deficient mice exhibit endophenotypes relevant to attention deficit hyperactivity disorder. Psychoneuroendocrinology. 2012;37:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15:352-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 79. | Davies W. Does steroid sulfatase deficiency influence postpartum psychosis risk. Trends Mol Med. 2012;18:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Elashoff M, Higgs BW, Yolken RH, Knable MB, Weis S, Webster MJ, Barci BM, Torrey EF. Meta-analysis of 12 genomic studies in bipolar disorder. J Mol Neurosci. 2007;31:221-243. [PubMed] |
| 81. | Strous RD, Maayan R, Weizman A. The relevance of neurosteroids to clinical psychiatry: from the laboratory to the bedside. Eur Neuropsychopharmacol. 2006;16:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Mihaljevic-Peles A, Jakovljevic M, Milicevic Z, Kracun I. Low arylsulphatase A activity in the development of psychiatric disorders. Neuropsychobiology. 2001;43:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Kumperscak HG, Paschke E, Gradisnik P, Vidmar J, Bradac SU. Adult metachromatic leukodystrophy: disorganized schizophrenia-like symptoms and postpartum depression in 2 sisters. J Psychiatry Neurosci. 2005;30:33-36. [PubMed] |
| 84. | Rauschka H, Colsch B, Baumann N, Wevers R, Schmidbauer M, Krammer M, Turpin JC, Lefevre M, Olivier C, Tardieu S. Late-onset metachromatic leukodystrophy: genotype strongly influences phenotype. Neurology. 2006;67:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Mevorah B, Frenk E, Müller CR, Ropers HH. X-linked recessive ichthyosis in three sisters: evidence for homozygosity. Br J Dermatol. 1981;105:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Trent S, Dean R, Veit B, Cassano T, Bedse G, Ojarikre OA, Humby T, Davies W. Biological mechanisms associated with increased perseveration and hyperactivity in a genetic mouse model of neurodevelopmental disorder. Psychoneuroendocrinology. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Trent S, Cassano T, Bedse G, Ojarikre OA, Humby T, Davies W. Altered serotonergic function may partially account for behavioral endophenotypes in steroid sulfatase-deficient mice. Neuropsychopharmacology. 2012;37:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Puri PK, Reddi DM, Spencer-Manzon M, Deak K, Steele SU, Mikati MA. Banding pattern on polarized hair microscopic examination and unilateral polymicrogyria in a patient with steroid sulfatase deficiency. Arch Dermatol. 2012;148:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | van Steensel MA, Vreeburg M, Engelen J, Ghesquiere S, Stegmann AP, Herbergs J, van Lent J, Smeets B, Vles JH. Contiguous gene syndrome due to a maternally inherited 8.41 Mb distal deletion of chromosome band Xp22.3 in a boy with short stature, ichthyosis, epilepsy, mental retardation, cerebral cortical heterotopias and Dandy-Walker malformation. Am J Med Genet A. 2008;146A:2944-2949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Ozawa H, Osawa M, Nagai T, Sakura N. Steroid sulfatase deficiency with bilateral periventricular nodular heterotopia. Pediatr Neurol. 2006;34:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Oades RD. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD). Prog Brain Res. 2008;172:543-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. 2011;75:151-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 95. | Kumar HB, Purushottam M, Kubendran S, Gayathri P, Mukherjee O, Murthy AR, Ghosh S, Chandra P, Reddy YC, Benegal V. Serotonergic candidate genes and puerperal psychosis: an association study. Psychiatr Genet. 2007;17:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Pytliak M, Vargová V, Mechírová V, Felšöci M. Serotonin receptors - from molecular biology to clinical applications. Physiol Res. 2011;60:15-25. [PubMed] |
| 97. | O’Neil RT, Emeson RB. Quantitative analysis of 5HT(2C) receptor RNA editing patterns in psychiatric disorders. Neurobiol Dis. 2012;45:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2012;42:2039-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 99. | Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 100. | Li PK, Rhodes ME, Jagannathan S, Johnson DA. Reversal of scopolamine induced amnesia in rats by the steroid sulfatase inhibitor estrone-3-O-sulfamate. Brain Res Cogn Brain Res. 1995;2:251-254. [PubMed] |
| 101. | Ciobanu LC, Luu-The V, Martel C, Labrie F, Poirier D. Inhibition of estrone sulfate-induced uterine growth by potent nonestrogenic steroidal inhibitors of steroid sulfatase. Cancer Res. 2003;63:6442-6446. [PubMed] |
| 102. | Johnson DA, Rhodes ME, Boni RL, Li PK. Chronic steroid sulfatase inhibition by (p-O-sulfamoyl)-N-tetradecanoyl tyramine increases dehydroepiandrosterone sulfate in whole brain. Life Sci. 1997;61:PL 355-359. [PubMed] |
| 103. | Rhodes ME, Li PK, Burke AM, Johnson DA. Enhanced plasma DHEAS, brain acetylcholine and memory mediated by steroid sulfatase inhibition. Brain Res. 1997;773:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 104. | Johnson DA, Wu T, Li P, Maher TJ. The effect of steroid sulfatase inhibition on learning and spatial memory. Brain Res. 2000;865:286-290. [PubMed] |
| 105. | Babalola PA, Fitz NF, Gibbs RB, Flaherty PT, Li PK, Johnson DA. The effect of the steroid sulfatase inhibitor (p-O-sulfamoyl)-tetradecanoyl tyramine (DU-14) on learning and memory in rats with selective lesion of septal-hippocampal cholinergic tract. Neurobiol Learn Mem. 2012;98:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Klinkenberg I, Sambeth A, Blokland A. Acetylcholine and attention. Behav Brain Res. 2011;221:430-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 107. | Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566-607. [PubMed] |
| 108. | Lykkesfeldt G, Bennett P, Lykkesfeldt AE, Micic S, Møller S, Svenstrup B. Abnormal androgen and oestrogen metabolism in men with steroid sulphatase deficiency and recessive X-linked ichthyosis. Clin Endocrinol (Oxf). 1985;23:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Traupe H, Happle R. Clinical spectrum of steroid sulfatase deficiency: X-linked recessive ichthyosis, birth complications and cryptorchidism. Eur J Pediatr. 1983;140:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Axt-Gadermann M, Schlichting M, Küster W. Male-pattern baldness is common in men with X-linked recessive ichthyosis. Dermatology. 2003;207:308-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 111. | Gånemo A, Sjöden PO, Johansson E, Vahlquist A, Lindberg M. Health-related quality of life among patients with ichthyosis. Eur J Dermatol. 2004;14:61-66. [PubMed] |
| 112. | Qureshi A, Thaver D. Cross sectional review of children with ADHD presenting to an outpatient psychiatric institute in Pakistan. J Pak Med Assoc. 2003;53:441-443. [PubMed] |
| 113. | Vasconcelos MS, Sampaio AS, Hounie AG, Akkerman F, Curi M, Lopes AC, Miguel EC. Prenatal, perinatal, and postnatal risk factors in obsessive-compulsive disorder. Biol Psychiatry. 2007;61:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 114. | Geller DA, Wieland N, Carey K, Vivas F, Petty CR, Johnson J, Reichert E, Pauls D, Biederman J. Perinatal factors affecting expression of obsessive compulsive disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2008;18:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 115. | Raynor MD, Oates MR. The psychology and psychopathology of pregnancy and childbirth. Myles Textbook for Midwives. 14th ed. Edinburgh: Churchill Livingstone 2003; 653-671. |
| 116. | Foster PA, Reed MJ, Purohit A. Recent developments of steroid sulfatase inhibitors as anti-cancer agents. Anticancer Agents Med Chem. 2008;8:732-738. [PubMed] |
| 117. | Palmieri C, Januszewski A, Stanway S, Coombes RC. Irosustat: a first-generation steroid sulfatase inhibitor in breast cancer. Expert Rev Anticancer Ther. 2011;11:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Purohit A, Fusi L, Brosens J, Woo LW, Potter BV, Reed MJ. Inhibition of steroid sulphatase activity in endometriotic implants by 667 COUMATE: a potential new therapy. Hum Reprod. 2008;23:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, Wilson RH, Stanczyk FZ, Dobbs N, Kulinskaya E. Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res. 2006;12:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 120. | Bukvic N, Cesarano C, Ceccarini C, Bruno M, Lipsi MR, Gallicchio MG, Carboni MA, Valente L, Cotoia G, Antonetti R. Characterization of the first adult de novo case of 46,X,der(Y)t(X; Y)(p22.3; q11.2). Gene. 2013;513:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 121. | Sismani C, Anastasiadou V, Kousoulidou L, Parkel S, Koumbaris G, Zilina O, Bashiardes S, Spanou E, Kurg A, Patsalis PC. 9 Mb familial duplication in chromosome band Xp22.2-22.13 associated with mental retardation, hypotonia and developmental delay, scoliosis, cardiovascular problems and mild dysmorphic facial features. Eur J Med Genet. 2011;54:e510-e515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |