Published online Nov 6, 2016. doi: 10.5527/wjn.v5.i6.497
Peer-review started: May 26, 2016
First decision: June 17, 2016
Revised: August 8, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: November 6, 2016
To evaluate incidence, risk factors and treatment outcome of BK polyomavirus nephropathy (BKVN) in a cohort of renal transplant recipients in the Auckland region without a formal BK polyomavirus (BKV) surveillance programme.
A cohort of 226 patients who received their renal transplants from 2006 to 2012 was retrospectively reviewed.
Seventy-six recipients (33.6%) had a BK viral load (BKVL) test and 9 patients (3.9%) developed BKVN. Cold ischaemia time (HR = 1.18, 95%CI: 1.04-1.35) was found to be a risk factor for BKVN. Four recipients with BKVN had complete resolution of their BKV infection; 1 recipient had BKVL less than 625 copies/mL; 3 recipients had BKVL more than 1000 copies/mL and 1 had graft failure from BKVN. BKVN has a negative impact on graft function [median estimated glomerular filtration rate (eGFR) 22.5 (IQR 18.5-53.0) mL/min per 1.73 m2, P = 0.015), but no statistically significant difference (P = 0.374) in renal allograft function was found among negative BK viraemia group [median eGFR 60.0 (IQR 48.5-74.2) mL/min per 1.73 m2), positive BK viraemia without BKVN group [median eGFR 55.0 (IQR 47.0-76.0) mL/min per 1.73 m2] and unknown BKV status group [median eGFR 54.0 (IQR 43.8-71.0) mL/min per 1.73 m2]. The incidence and treatment outcomes of BKVN were similar to some centres with BKV surveillance programmes.
Recipients with BVKN have poorer graft function. Although active surveillance for BKV has been shown to be effective in reducing incidence of BKVN, it should be tailored specifically to that transplant centre based on its epidemiology and outcomes of BKVN, particularly in centres with limited resources.
Core tip: A retrospective analysis of 226 patients from Auckland, New Zealand found BK polyomavirus (BKV) as an uncommon cause of graft loss. Renal units without a formal BKV surveillance programme showed a similar incidence and outcomes for BK polyomavirus nephropathy (BKVN) to centres with an active screening programme. When designing a cost effective screening programme for BKV infection, it should be centre specific in relation to the units immunosuppression and monitoring protocol, epidemiology and outcomes of BKVN.
- Citation: Hsiao CY, Pilmore HL, Zhou L, de Zoysa JR. Outcomes of renal transplant recipients with BK virus infection and BK virus surveillance in the Auckland region from 2006 to 2012. World J Nephrol 2016; 5(6): 497-506
- URL: https://www.wjgnet.com/2220-6124/full/v5/i6/497.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i6.497
Since the first discovery of BK polyomavirus (BKV) isolated from the urine of a renal allograft recipient with ureteric obstruction in 1971[1], BKV infection has emerged as an important cause of renal allograft dysfunction. In the modern era, with the use of more potent immunosuppressive agents, BKV nephropathy (BKVN) has resulted in a significant rate of graft loss[2].
Seroprevalence of BKV in immunocompetent adults ranges from 65% to 90% and BKV remains latent predominately in the urinary tract[3]. Immunosuppression, inflammation and insufficient anti-viral immune responses play an integral role in the reactivation and replication of BKV and progression to BKVN in renal transplant recipients[4]. Dendritic cell deficiency, seronegativity for BKV and impaired BKV-specific T-cell response are found to be associated with BK viraemia[5-8]. Other possible risk factors for BK viraemia include a long cold ischaemia time (CIT)[9], acute rejection[10,11], placement of a ureteric stent[12,13], human leukocyte antigen (HLA) mismatch[11,14], lymphocyte-depleting agents[15-18], tacrolimus[19,20] and steroids[16,19,20]. Given the complexity in the pathogenesis of BKVN, the intensity of immunosuppression may not be solely responsible for the development of BKVN and it may not be appropriate to generalise these predisposing risk factors in all renal transplant recipients. However, as the immunosuppressive burden appears a significant risk factor for the development of BK polyomavirus, it is possible that the immunosuppression regimen used accounts at least in part for the substantial variation in the prevalence of BK viruria (30%-62%), BK viraemia (11.5%-20%) and BKVN (1%-10%) among transplant centres[10,12,21,22].
No effective pharmacological treatment has consistently emerged for the treatment of BKV infection apart from a reduction of immunosuppressants[23,24]. Many investigators have recommended screening BK viraemia and pre-emptive reduction in immunosuppressants as a strategy to preserve graft function and reduce the risk of BKVN occurring[25-28]. However, a screening programme may not be suitable in a transplant centre with a low rate of BVKN[29].
We conducted a retrospective review of renal transplant recipients from our region, where no lymphocyte depleting induction treatment is used and the predominant calcineurin inhibitor utilised is Ciclosporin, to determine the incidence of BKVN and/or BK viraemia and to evaluate the characteristics that are potentially associated with the development of BKVN and related treatment outcome.
We searched through the Auckland Renal Transplant Group’s database to identify patients who received renal transplants from January 2006 to December 2012. Patients resident outside the Auckland region were not included in this retrospective review. We excluded those who died or had primary graft failure within 1 mo of receiving a renal allograft. Patients’ clinical notes with clinical and demographic data were obtained from the clinical electronic portal system, Concerto® and 3M® Health Information Systems, with data censored for 31 December 2013.
Testing for serum BKV viral load is performed at a single laboratory, LabPlus™, in the Auckland region. The laboratory does not routinely perform BKV viral load in urine samples, due to the high prevalence of positive BK viruria in renal transplant recipients. Recipients are considered to have BK viraemia for any level of viral load. Testing for serum creatinine is performed at both hospital and community laboratories in the Auckland region. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation[30]. The recipients’ renal biopsies performed in the Auckland region were processed and interpreted at a single laboratory: LabPlus™.
There is no formal screening programme among the three renal units in the Auckland region. One unit routinely screened for BKV from the year 2012. One of these hospitals trialled screening for BKV in 2009, but halted this strategy due to cost, and BK viral load (BKVL) test is performed only if there is a biopsy-proven BKVN. The third unit checks for BKV if there is a clinical indication or a biopsy-proven BKVN.
Basiliximab was routinely used for induction from August 2010. All male recipients were given Ciclosporin as their maintenance calcineurin inhibitor, but female recipients were given a choice of either Tacrolimus or Ciclosporin. The tacrolimus trough concentration in each recipient was kept between 10 to 15 μg/L for the first 2 mo after transplant, and then the target concentration was kept between 5 to 10 μg/L. The Ciclosporin (2 h post dose) target concentration was 1700 μg/L for the first month after transplant and 1500 μg/L for the second month. The target concentration was then gradually reduced to equal to or less than 800 μg/L after 12 mo of transplantation. Every recipient received Mycophenolate mofetil (MMF) 1 g twice daily if on Ciclosporin or 750 mg twice daily if on Tacrolimus. Concentration monitoring for MMF is not routinely performed. All recipients received Methylprednisolone 1 g at induction followed by prednisone as maintenance. The prednisone dose was 30 mg daily for all recipients and it was tapered down to 7.5 mg daily or lower at months 4 post-transplant.
Recipients who have acute rejection Banff grade I receive Methylprednisolone 500 mg daily for 3 d. For Banff grade II, III and steroid-resistant rejections, patients receive lymphocyte-depleting agents. Biopsy-proven rejection in the presence of therapeutic Ciclosporin concentrations necessitates a conversion to Tacrolimus unless contraindicated. The target Tacrolimus concentration is maintained between 7 to 10 μg/L.
A protocol biopsy at 3 mo after transplantation is performed in all renal transplant recipients. Patients with a > 20% decline in graft function without a clear cause and all reversible non-renal causes excluded undergo renal biopsy.
SPSS version 22 was used to perform statistical analysis. Results are expressed as numbers (percentages), median (interquartile range Q1-Q3) and mean (± SD) unless otherwise stated. The 95%CIs are based on exact confidence limits. Data were compared by χ2 test, Fisher’s exact test, non-parametric Mann-Whitney U test or Kruskal-Wallis test as appropriate. Cox regression was used to identify possible risk factors for BKVN. Those who died, developed graft failure unrelated to BKVN and were transferred to outside of the Auckland region were censored in this model.
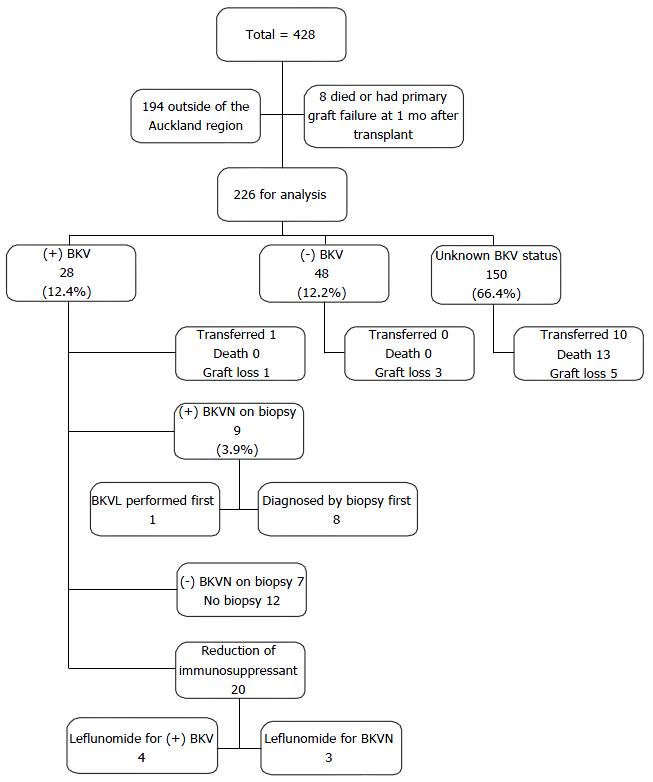
Four hundred and twenty-eight patients underwent renal transplantation between January 2006 and December 2012. After excluding 194 patients from outside of the Auckland region and 8 patients who died or developed primary graft failure at one month after transplant, 226 were included in the study (Figure 1).
Seventy-six recipients were tested for BKV (33.6%) at any point in time over the study period. Twenty-eight patients of 76 tested patients had BK viraemia (36.8%). Twenty of these 28 patients were managed with a reduction of their immunosuppressants; by reducing or stopping MMF and maintaining Tacrolimus trough concentration and Ciclosporin trough concentration between 4-6 μg/L and 100-150 μg/L, respectively, depending on BKVLs’ responses. Seven patients received Leflunomide concurrently; one BKVN recipient was given additional Ciprofloxacin as the BKVL failed to decline despite reducing immunosuppressants, and another BKVN recipient also received Ciprofloxacin and subsequently intravenous Immunoglobulin due to persistent high level of BK viraemia, worsening graft dysfunction and a repeat graft biopsy showed features of acute rejection in addition to BKVN. The remaining 8 BK viraemic recipients did not have a reduction of immunosuppressants due to low viral loads (all less than 1250 copies/mL).
Of these 28 patients, 16 patients had transplant biopsies for renal allograft dysfunction. Nine patients had biopsy-proven BKV nephropathy equivalent to an incidence of 11.8% (95%CI: 5.6%-21.3%) of the cohort tested for BKV viral load (9/76) or 4.0% (95%CI: 1.8%-7.4%) of the entire cohort (9/226). Eight of these nine patients were tested for BKVLs after their transplant biopsies diagnosed BKVN. The other patient with BKVN had the transplant biopsy and serum BKVL requested concurrently as part of investigation of graft dysfunction. The remaining seven transplant recipients with transplant biopsies for graft dysfunction did not have BKVN. The other 12 recipients did not have transplant biopsies as their graft functions were stable, and 7 of these 12 recipients had their immunosuppression reduced.
Three (33.3%) of the recipients with BKVN did not have acute rejection prior to the diagnosis of BKVN. The other 6 recipients (66.7%) had at least one episode of biopsy proven acute rejection requiring a pulse methylprednisolone course prior to the development of BKVN. Ten of the 19 recipients (53.7%) with BK viraemia did not have acute rejection prior to the diagnosis of BK viraemia and the others (47.3%) had at least one acute rejection prior.
When comparing features between recipients with BKVN and without BKVN, we found that BKVN was more common in Māori, Pacific Islanders and Asians than in Europeans (European 1.5%% vs Asian 9.3% vs Māori and Pacific Islanders 7.4%, P = 0.038). The renal allografts of the recipients that developed BKVN were all from deceased donors (P = 0.005). The BKVN group had a longer CIT (median 19 h vs 7 h, P = 0.001). The group was also more likely to have at least 1 episode of acute rejection at any time point, though it was not statistically significant (P = 0.069). There was no significant difference observed in age, gender, co-morbidity, dialysis vintage, HLA mismatch, Tacrolimus, Basiliximab induction, or use of lymphocyte-depleting agents for acute rejection between the positive (+) BKVN and negative (-) BKVN groups (Table 1).
| (-) BKVN (n = 217) | (+) BKVN (n = 9) | P value | (+) BKV (n = 19) | P value | |
| Age (yr) | 48 (35.3-56.8) | 55 (34.9-60.5) | NS | 50 (27.1-60) | NS |
| Gender (female) | 92 (42.3) | 3 (33.3) | NS | 6 (31.6) | NS |
| Ethnicity | |||||
| European | 131 (98.5) | 2 (1.5) | 0.038 | 11 (57.9) | 0.191 |
| Asian | 32 (90.7) | 3 (9.3) | 4 (21.1) | ||
| Māori and Pacific Islander | 54 (92.6) | 4 (7.4) | 4 (21.1) | ||
| Cause of ESKF | |||||
| Glomerulonephritis | 91 (41.9) | 5 (55.6) | NS | 8 (42.1) | NS |
| Diabetes mellitus | 26 (12.0) | 2 (22.2) | 2 (10.5) | ||
| Others | 55 (46.1) | 2 (22.2) | 9 (47.4) | ||
| Co-morbidity | |||||
| Diabetes mellitus | 26 (11.9) | 2 (22.2) | NS | 2 (10.5) | NS |
| Hypertension | 130 (59.9) | 5 (55.5) | NS | 12 (63.2) | NS |
| Cardiac disease | 31 (14.2) | 2 (22.2) | NS | 3 (15.8) | NS |
| Dialysis vintage (yr) | 2 (0.2-5) | 5 (1-6) | NS | 2.5 (0-5) | NS |
| Donor source (deceased) | 118 (54.3) | 9 (100) | 0.005 | 9 (47.4) | 0.01 |
| HLA mismatch | 3 (2-4) | 4 (2.5-4) | NS | 3.5 (2-5) | NS |
| Basiliximab induction | 95 (43.7) | 3 (33.3) | NS | 13 (68.4) | NS |
| Tacrolimus | 92 (42.3) | 5 (55.6) | NS | 10 (52.6) | NS |
| Cold ischaemia time (h) | 7 (4-15) | 19 (14.2-20.4) | 0.001 | 5.4 (4-12.5) | < 0.0001 |
| AR at any time point | |||||
| 0 episode | 144 (66.4) | 3 (33.3) | 0.069 | 9 (47.4) | NS |
| ≥ 1 episode(s) | 73 (33.6%) | 6 (66.7) | 10 (52.6) | ||
| AR before known BKV/BKVN status | |||||
| 10 → 0 episode | - | 3 (33.3) | 10 (52.6) | NS | |
| 11 →≥ 1 episode(s) | - | 6 (66.7) | 9 (47.6) | ||
| Thymoglobulin for acute rejection | 33 (15.2) | 0 (0) | NS | 5 (26.3) | NS |
There was no statistical difference in ethnicity, acute rejection at any time point and other demographics when comparing the BKVN group and the (+) BKV group (recipients with only BK viraemia and no biopsy-proven BKVN). The (+) BKV group had fewer deceased donors (47.4%, P = 0.01) and shorter CIT (median 5.4 h, P < 0.0001) than patients who developed BKV nephropathy. BK viraemic recipients (47.4%) and recipients with BKVN (66.7%) were more likely to have acute rejection prior to the diagnosis of BKVN and BK viraemia as opposed to recipients without BK viraemia (27.1%, P = 0.048).
In a univariable Cox regression analysis, Asian recipients had a greater risk of developing BKVN compared with European recipients (unadjusted HR = 6.36, 95%CI: 1.96-38.16, P = 0.043), but it was not seen in Māori and Pacific islands recipients (unadjusted HR = 4.75, 95%CI: 0.87-25.94, P = 0.072) (Table 2). Because CIT is dependent on donor source, donor source was not used in the analysis. Recipients with longer CIT had a higher risk of developing BKVN (unadjusted HR = 1.18, 95%CI: 1.06-1.32, P = 0.003). While acute rejection appeared to be associated with BKVN, this did not reach statistical significance (unadjusted HR = 3.72, 95%CI: 0.93-14.91, P = 0.063).
| Crude HR (+) BKVN | 95%CI | P value | Adjusted HR (+) BKVN | 95%CI | P value | |
| Gender (female) | 0.66 | 0.16-2.66 | NS | |||
| Ethnicity (reference: European) | ||||||
| Asian | 6.36 | 1.06-38.16 | 0.043 | 3.731 | 0.61-22.92 | 0.154 |
| Māori/Pacific Islander | 4.75 | 0.87-25.94 | 0.072 | 2.631 | 0.45-15.25 | 0.279 |
| Co-morbidity | ||||||
| Diabetes mellitus | 2.06 | 0.42-9.94 | NS | |||
| Basiliximab induction | 0.75 | 0.18-3.03 | NS | |||
| Tacrolimus | 1.64 | 0.44-6.11 | NS | |||
| Thymoglobulin for rejection | 0.03 | 0.00-88.65 | NS | |||
| Cold ischaemia time | 1.18 | 1.06-1.32 | 0.003 | 1.181 | 1.04-1.35 | 0.009 |
| Acute rejection (≥ 1 episode)2 | 3.72 | 0.93-14.91 | 0.063 | 4.051 | 0.99-16.53 | 0.051 |
| HLA mismatch | 1.15 | 0.75-1.77 | NS |
We included only the variables that had P value < 0.1 in a multivariable model. Ethnicity was not found to be significant, and Longer CIT was the only risk factor for BKVN (HR = 1.18, 95%CI: 1.05-1.39, P = 0.009).
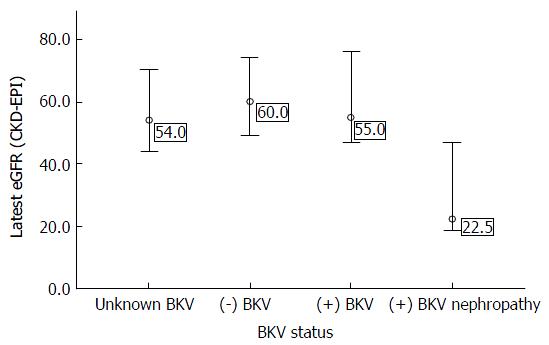
Renal allograft function in the BKVN group was significantly lower comparing with those in the other BKV status groups (P = 0.015), and the median graft function of the recipients who were never checked for BK viraemia was similar to those with and without BK viraemia using the non-parametric Kruskal-Wallist test (P = 0.374) (Figure 2). After controlling for those factors (age at transplant, comorbidity, donor source, HLA mismatch, acute rejection, Basiliximab induction, calcineurin inhibitor and eGFR at 1 mo after transplant) that could potentially affect graft function, the mean eGFR of the recipients with BKVN (taken just before the censored date or before recipients were transferred, but those who developed graft failure were not included) was still 17.0 mL/min per 1.73 m2 (95%CI: -32.5 to -1.5 mL/min per 1.73 m2, P = 0.032) lower than that of those without BKVN. However, no significant impact on graft function was found in recipients with only BK viraemia (-4.1 mL/min per 1.73 m2, P = 0.464) and unknown BKV status (-1.1 mL/min per 1.73 m2, P = 0.754) comparing with the negative BK viraemic recipients.
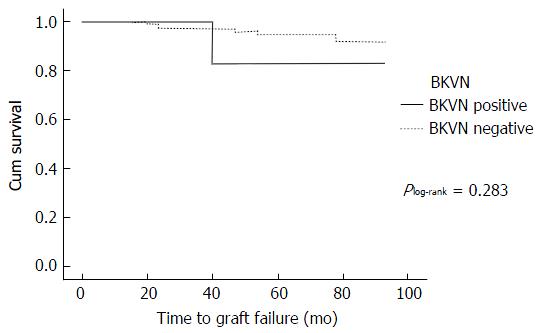
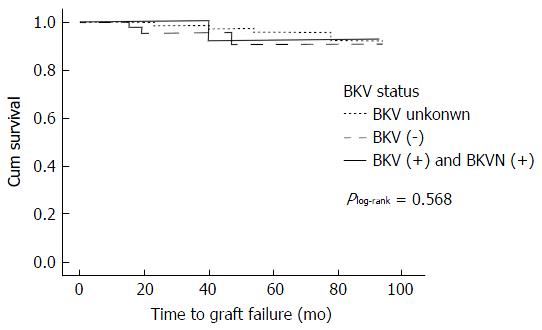
One of the 9 recipients (11.1%) with BKVN had graft failure compared to 8 (3.7%) of those without, but there was no statistical difference found using a log-rank test in the Kaplan-Meier survival analysis (Plog-rank = 0.283) (Figure 3). Similarly, no difference was found between those with known and unknown BKV status (0.92 for positive BKV and BKVN, 0.90 for negative BKV, and 0.92 for unknown BKV status, Plog-rank = 0.568) (Figure 4).
For those diagnosed with BKVN, BKVLs were undetectable in 4 recipients and the BKVL was less than 625 copies/mL in one recipient at the time of censoring. The other 3 patients still had persistent BK viraemia but viral loads were declining at the end of study period (Table 3). One patient developed graft failure due to BKVN, but it is important to note that this recipient’s graft function was poor prior to the diagnosis of BKVN (eGFR 23-27 mL/min per 1.73 m2) with no clear explanation despite 4 graft biopsies that did not show any significant abnormality. BKVLs of the recipients with BK viraemia were either undetectable or declining during the study period. None of these 19 patients, including six of them with BKVLs more than 104 copies/mL, progressed to BKVN over the study period.
| Recipients with BKVN | BKVL at diagnosis | eGFR at 1 mo | eGFR at CD | BKVL outcome1 | Time to outcome1 | Acute rejection (post reduction of IS) |
| 1 | 1100000 | 48.7 | 73 | Undetectable | 28 mo | No |
| 2 | 3250 | 17.8 | 22 | Undetectable | 44 mo | Yes (at 30 mo) |
| 3 | 364700 | 36.9 | 23 | Undetectable | 31 mo | Yes (at 2 mo) |
| 4 | 265650 | 21.5 | 59 | Undetectable | 17 mo | No |
| 5 | 47225 | 23 | 18 | < 625 | 7 mo | No |
| 6 | 1794502 | 57 | 35 | 3725 | 59 mo | No |
| 7 | 59736502,3 | 33.4 | 20 | 41175 | 12 mo | No |
| 8 | 295608752,3,4 | 50.2 | 16 | 30425 | 8 mo | No |
| 9 | 56125 | 27.9 | Graft failure | Graft failure | 21 mo | No |
Of the patients who had a reduction in immunosuppression none of the 11 patients with BK viraemia had acute rejection, while two of the 9 recipients with BKVN developed an acute T-cell mediated rejection grade 1B at 30 mo and 2 mo after their immunosuppressive therapy was reduced. The acute rejections were successfully treated with intravenous pulse methylprednisolone, and their calcineurin inhibitors were already switched to Tacrolimus from previous acute rejection prior to the diagnosis of BKVN.
This retrospective study has demonstrated that only one patient had graft failure due to BKVN in our cohort of patients. We identified 28 patients with BKV infection with a rate of 36.8% in the cohort that was screened. However, this has likely overestimated the incidence as 150 patients (66.4%) were not tested for BK viraemia. When considering the entire cohort the rate of BKV was 12.4% (28 of 226). The incidence of biopsy proven BKVN was 11.8% (9/76). It is important to note that it is unlikely that any patient with clinically significant BKV did not undergo testing due to the regional protocol to perform renal biopsy. However, it is possible that early BKVN could be missed by renal biopsy due to its focal nature[25]. Using the overall cohort we noted an incidence of 4.0% (9/226) which is comparable to that of other transplant centres with an active BKV screening programmes (0.8% to 6.4%)[11,26-28,31-33].
A long CIT was identified as a potential risk factor for BKVN in our study, and is known to cause severe ischaemia-reperfusion injury resulting in intragraft inflammation, which in turn is found to stimulate BK polyomavirus DNA replication[34,35]. Acute rejection results in tubulointerstitial inflammation and typically leads to also an increased burden of immunosuppression. However, interestingly in our cohort, acute rejection was not found to be a risk factor for progressing to BKVN. Although others have demonstrated an association between Tacrolimus use and BKVN, Tacrolimus was not found to be associated with BKVN in our cohort. Despite lymphocyte-depleting agents were used for severe acute rejection episodes in this cohort, our analysis did not demonstrate more recipients with BK viraemia and BKVN. Other studies have also failed to show any correlation between lymphocyte-depleting agents given at induction and BKV infection[26,28].
Because of the difficulty in predicting BKVN in transplant recipients, a latent period from BK viraemia to the development of BKVN, and possible sampling errors in diagnosing early BKVN due to its focal nature[25], screening of the blood or urine for BKV infection and pre-emptive reduction of immunosuppressants when BKV is detected have been shown to reduce, the risk of the development of BKVN (0.8%-1.3%)[27,28,32,33]. Interestingly, some centres report that despite having a BKV screening programme in the literature, their incidence of BKVN is greater than that seen in our cohort (4.2%-6.4%)[11,26,31], This includes one centre that screened for decoys cells in urine fortnightly for the first 3 mo, then at 6 and 12 mo and yearly after transplant[31]. It appears that frequent monthly BKV monitoring is essential in early detection of BK viraemia and intervention resulting in the lower incidences of BKVN described in the abovementioned studies, with one centre that performed a cheaper urine cytology screening fortnightly from 0 to 3 mo after transplant, monthly from 3 to 6 mo and then every 2 mo from 6 to 12 mo[33].
Because all patients with a > 20% decline in renal function undergo routine biopsy and all patients undergo a protocol biopsy at 3 mo after transplantation in our study cohort, we postulate that a large proportion (66.4%) of our cohort with unknown BKV status had no episodes of graft dysfunction due to BKVN. Bohl and his co-investigators looked at other studies and commented that BKVLs exceeding 104 copies/mL only have a positive predictive value of 50%-85% for diagnosing BKVN[36]. BKVLs that are less than 104 copies/mL do not require intervention[28,37]. This is reflected not only on the recipients who never had any BKVL tests, but also on the eight patients with low BKVLs who did not progress to BKVN even without a reduction of immunosuppressants and further BKV monitoring.
BKVN impacted on graft function; one patient lost their allograft and the other eight patients had a mean eGFR more than over 18 mL/min worse than patients without BKVN. This finding of poorer graft function from BKVN is also found in a prospective study that adopted a rigorous monthly screening programme - the mean eGFR of recipients with BKVN (39.0 ± 14.3 mL/min per 1.73 m2) was lower than that of those without BKVN (52.3 ± 19.9 mL/min per 1.73 m2), though there was no graft failure due to BKVN[28].
Frequent monthly BKV monitoring and pre-emptive reduction of immunosuppression have been shown to be beneficial in reducing the risk of BKVN occurring. This strategy is effective in improving overall graft outcomes in renal transplant recipients as there is no definitive medical therapy for BKVN with graft failure still occurring. However, this approach may be cost prohibitive for those resource restricted centres especially where a low incidence of BKVN is identified[29]. Screening for decoy cells in the urine first may be a cheaper option[33], but there is a high prevalence rate of BK viruria even in immunocompetent adults and not all centres have resources to perform this test. Though cost saving can be achieved by reductions of immunosuppression as described in the literature[38,39], they may not necessarily cover the cost of screening for BKV if immunosuppressants are inexpensive. To reduce the financial burden by increasing the monitoring interval to every 3 mo or longer, it may not reduce the incidence of BKVN as seen in the other studies. Nevertheless, either screening approach may not necessarily preserve graft function for those with BKVN. It is also interesting to see that the group with unknown BKV status in this cohort had a similar median eGFR comparing to those without BK viraemia, thus questioning the necessity of screening in this context. Our study is likely under-powered and increasing differences among these groups would likely have been observed in a large sample size. Targeting only those recipients who have significant predisposing risk factors could potentially reduce screening cost, but there is no consistency in what these risk factors are in the literature, thereby failing to identify BKVN cases early. Another cost saving option would be to identify recipients with positive BK viraemia early by performing an intensive monthly BKV screening only in the first 3 mo of renal transplant when the degree of immunosuppression is the greatest followed by a 3 mo screening until 12 mo after transplant. Therefore, when designing a BKV surveillance programme, it should be centre specific by taking the epidemiology of BKV infection, immunosuppression and monitoring protocols and related costs in a transplant centre into consideration to make it viable and cost effective.
There are several limitations in this retrospective study. Due to the lack of a comprehensive screening program we may have underestimated the incidence of BKVN. With our current approach of undertaking renal biopsy in all patients with a significant decline in renal function, it is unlikely that there are many patients with clinically significant BKVN that are not recognised. Interestingly, three patients with BKVLs of more than 160000 copies/mL did not have transplant biopsies due to stable graft function. Because of the selected cohort, the sizes of the comparative groups were small and there were only 9 recipients with BKVN which allow only a small number of variables to be included in the multivariable Cox regression model. As a result, the study is likely under-powered. In addition, there might be other risk factors for BKVN that were not identified, and tacrolimus and ciclosporin levels were not included in this study. Due to a large number of the recipients not tested for BKV, we cannot make effective comparisons, evaluate risk factors for BK viraemia or perform a cost analysis in this cohort.
A comprehensive BK virus surveillance program and reduction of immunosuppressive therapy is the recognised management strategy to reduce the risk of BKVN occurring, because BKVN significantly impairs graft function. This study highlights that in our cohort the incidence of BKVN and graft failure due to BKVN without a formal screening programme is low and comparable to some transplant centres that have a BKV surveillance programme. Long CIT is associated with BKVN. The risk factor for BKVN identified is not consistent with other studies suggesting intricacy in the pathogenesis of BKVN and different protocols adopted by various transplant centres. Though the outcomes of this study remain speculative particularly the incidence of BKVN due to the study’s limitations and it requires further validation in larger trials, it provides a similar perspective in BK virus screening to Kiberd’s and Smith’s studies[29,38]. Transplant centres should evaluate its immunosuppression and monitoring protocols, the epidemiology of BKV infection and related costs before designing a BKV surveillance programme to make it centre specific and cost effective.
Screening for BK viraemia is an important strategy in reducing the risk of BK polyomavirus nephropathy (BKVN) and requires monthly monitoring in the first year of transplant in order for a BK virus surveillance to be effective. Applying this surveillance strategy in any transplant centres may not necessarily produce the best possible outcomes due to resource constraints and a low incidence of BK virus infection. This retrospective study was performed to compare outcomes of BK virus infection in the Auckland region without a formal BK virus screening program with those in centres with a BK virus surveillance program reported in literature.
There are many risk factors, such as immunosuppressive burden, human leukocyte antigen mismatch, implicated in BK virus infection and the development of BKVN in renal transplant recipients. Therefore, immunosuppression and monitoring protocols play a role in BK virus infection. When designing a surveillance program for BK virus, the authors feel that immunosuppression and monitoring protocols, the epidemiology of BK virus infection and related costs should also be evaluated.
Outcomes of BKV infection, particularly BKVN, in this studied cohort are similar to some of transplant centres with a formal BK screening program that has less frequent testing for BKV.
Screening for BKV infection is important. Some transplant centres with limited resources may not afford frequent BKV testing or have capacity to perform BKV test. The study results suggest that a BKV screening program should be centre specific to be cost effective and achieve best possible outcomes.
This retrospective study looked at the incidence of BK viremia, BK nephropathy and graft outcomes among kidney transplant recipients in Auckland region. Study is well conducted and written clearly.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: New Zealand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P- Reviewer: Ciotti M, Hammes M, Milford DV, Sureshkumar KK, Zhang ZH S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 493] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, Gudat F, Mihatsch MJ, Thiel G. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 368] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, Miller E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 4. | Babel N, Volk HD, Reinke P. BK polyomavirus infection and nephropathy: the virus-immune system interplay. Nat Rev Nephrol. 2011;7:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Womer KL, Huang Y, Herren H, Dibadj K, Peng R, Murawski M, Shraybman R, Patton P, Clare-Salzler MJ, Kaplan B. Dendritic cell deficiency associated with development of BK viremia and nephropathy in renal transplant recipients. Transplantation. 2010;89:115-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Comoli P, Binggeli S, Ginevri F, Hirsch HH. Polyomavirus-associated nephropathy: update on BK virus-specific immunity. Transpl Infect Dis. 2006;8:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Hariharan S, Cohen EP, Vasudev B, Orentas R, Viscidi RP, Kakela J, DuChateau B. BK virus-specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am J Transplant. 2005;5:2719-2724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Binggeli S, Egli A, Schaub S, Binet I, Mayr M, Steiger J, Hirsch HH. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7:1131-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 903] [Cited by in F6Publishing: 873] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 11. | Hässig A, Roos M, Etter A, Bossart W, Müller N, Schiesser M, Wüthrich RP, Fehr T. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl Infect Dis. 2014;16:44-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, Torrence S, Schuessler R, Roby T, Gaudreault-Keener M. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 515] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 13. | Kayler L, Zendejas I, Schain D, Magliocca J. Ureteral stent placement and BK viremia in kidney transplant recipients. Transpl Infect Dis. 2013;15:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Awadalla Y, Randhawa P, Ruppert K, Zeevi A, Duquesnoy RJ. HLA mismatching increases the risk of BK virus nephropathy in renal transplant recipients. Am J Transplant. 2004;4:1691-1696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Dharnidharka VR, Cherikh WS, Neff R, Cheng Y, Abbott KC. Retransplantation after BK virus nephropathy in prior kidney transplant: an OPTN database analysis. Am J Transplant. 2010;10:1312-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Dadhania D, Snopkowski C, Muthukumar T, Lee J, Ding R, Sharma VK, Christos P, Bang H, Kapur S, Seshan SV. Noninvasive prognostication of polyomavirus BK virus-associated nephropathy. Transplantation. 2013;96:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Borni-Duval C, Caillard S, Olagne J, Perrin P, Braun-Parvez L, Heibel F, Moulin B. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation. 2013;95:1498-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Schold JD, Rehman S, Kayle LK, Magliocca J, Srinivas TR, Meier-Kriesche HU. Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22:626-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, Scheuermann EH, Klinger M, Russ G, Pescovitz MD. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13:136-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Manitpisitkul W, Drachenberg C, Ramos E, Munivenkatappa R, Philosophe B, Klassen D, Haririan A. Maintenance immunosuppressive agents as risk factors for BK virus nephropathy: a case-control study. Transplantation. 2009;88:83-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Ginevri F, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, Bodaghi S, Salotti V, Rinieri A, Botti G. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7:2727-2735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, Mihatsch MJ, Nickeleit V, Ramos E, Randhawa P. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 743] [Cited by in F6Publishing: 690] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 23. | Lee BT, Gabardi S, Grafals M, Hofmann RM, Akalin E, Aljanabi A, Mandelbrot DA, Adey DB, Heher E, Fan PY. Efficacy of levofloxacin in the treatment of BK viremia: a multicenter, double-blinded, randomized, placebo-controlled trial. Clin J Am Soc Nephrol. 2014;9:583-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89:1057-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:179-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 26. | Knight RJ, Gaber LW, Patel SJ, DeVos JM, Moore LW, Gaber AO. Screening for BK viremia reduces but does not eliminate the risk of BK nephropathy. Transplantation. 2013;96:e51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Alméras C, Vetromile F, Garrigue V, Szwarc I, Foulongne V, Mourad G. Monthly screening for BK viremia is an effective strategy to prevent BK virus nephropathy in renal transplant recipients. Transpl Infect Dis. 2011;13:101-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Elfadawy N, Flechner SM, Liu X, Schold J, Tian D, Srinivas TR, Poggio E, Fatica R, Avery R, Mossad SB. The impact of surveillance and rapid reduction in immunosuppression to control BK virus-related graft injury in kidney transplantation. Transpl Int. 2013;26:822-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Kiberd BA. Screening to prevent polyoma virus nephropathy: a medical decision analysis. Am J Transplant. 2005;5:2410-2416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [PubMed] [Cited in This Article: ] |
| 31. | Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, Mayr M. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10:2615-2623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 32. | Zaman RA, Ettenger RB, Cheam H, Malekzadeh MH, Tsai EW. A novel treatment regimen for BK viremia. Transplantation. 2014;97:1166-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Chakera A, Dyar OJ, Hughes E, Bennett S, Hughes D, Roberts IS. Detection of polyomavirus BK reactivation after renal transplantation using an intensive decoy cell surveillance program is cost-effective. Transplantation. 2011;92:1018-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Liang B, Tikhanovich I, Nasheuer HP, Folk WR. Stimulation of BK virus DNA replication by NFI family transcription factors. J Virol. 2012;86:3264-3275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Atencio IA, Shadan FF, Zhou XJ, Vaziri ND, Villarreal LP. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993;67:1424-1432. [PubMed] [Cited in This Article: ] |
| 36. | Bohl DL, Brennan DC. BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol. 2007;2 Suppl 1:S36-S46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Sood P, Senanayake S, Sujeet K, Medipalli R, Zhu YR, Johnson CP, Hariharan S. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94:814-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Smith F, Panek R, Kiberd BA. Screening to prevent polyoma virus nephropathy in kidney transplantation: a cost analysis. Am J Transplant. 2009;9:2177-2179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Laskin BL, Goebel J. Cost-efficient screening for BK virus in pediatric kidney transplantation: a single-center experience and review of the literature. Pediatr Transplant. 2010;14:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |