Revised: October 26, 2012
Accepted: January 5, 2013
Published online: February 6, 2013
Podocytes covering the glomerular basement membrane over the glomerular capillary consist of three morphologically and functionally different segments, the cell body, major processes and extending finger-like foot processes (FPs). The FPs of neighboring podocytes are connected by a continuous adherent junction structure named the slit diaphragm (SD). The extracellular SD is linked to the intracellular, a highly dynamic, cytoskeleton through adaptor proteins. These adaptor proteins, such as CD2-associated protein, zonula occludens 1, β-catenin, Nck and p130Cas, located at the intracellular SD insertion area near lipid rafts, have important structural and functional roles. Adaptor proteins in podocytes play important roles as a structural component of the podocyte structure, linking the SD to the cytoskeletal structure and as a signaling platform sending signals from the SD to the actin cytoskeleton. This review discusses the roles of adaptor proteins in the podocyte cytoskeletal structure and signaling from the SD to the actin cytoskeleton.
- Citation: Ha TS. Roles of adaptor proteins in podocyte biology. World J Nephrol 2013; 2(1): 1-10
- URL: https://www.wjgnet.com/2220-6124/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i1.1
Podocytes covering the glomerular basement membrane (GBM) over the glomerular capillary consist of three morphologically and functionally different segments, the cell body, major processes and extending interdigitating foot processes (FPs). The FPs of podocytes are connected by a continuous adherent junction structure named the slit diaphragm (SD), forming filtration pores. The extracellular SD is linked to the intracellular actin-based cytoskeleton through adaptor proteins, such as CD2-associated protein (CD2AP), zonula occludens (ZO)-1, β-catenin, Nck and p130Cas, located at the intracellular SD insertion area near lipid rafts[1-7]. Here, I discuss the structural and functional roles of adaptor proteins in podocyte cytoskeletal structure and signaling.
As the glomerular capillary wall functions as an efficient and selective barrier that allows a high flow rate of filtration for plasma water and small solutes, the glomerular capillary wall should have a strong but selectively permeable filtration membrane and cell-to-cell junction[1-3]. The glomerular tuft, a network of tangled capillaries, is composed of three cell types: endothelial cells at the inside of the capillary, podocytes on the outside of the capillary, and mesangial cells supporting the capillary loops. Together with the GBM, the endothelium and podocytes form the filtration barrier[1-5].
Podocytes are highly differentiated epithelial cells that cover the outer layer of the GBM, playing a crucial role in the regulation of glomerular function. Podocytes consist of three morphologically and functionally different segments, including the cell body, major processes and extending FPs. The FPs of neighboring podocytes alternatively interdigitate, leaving between them the filtration slits that are bridged by an extracellular zipper-like structure, known as the SD[1-5]. The SD is a modified adherens junction with a filtration slit that is 25-60 nm wide and contains nephrin, P-cadherin, NEPH1, FAT etc. so far[3]. SD serves as a size-selective barrier and is linked to the actin-based cytoskeleton by adaptor proteins, including CD2AP, ZO-1, β-catenin, podocin, etc.[1-5].
The podocyte consists of a cell body, major processes and interdigitating FPs. The cytoskeleton of both the cell body and major processes of podocytes are formed by microtubules and intermediate filaments, such as vimentin, talin and vinculin. Intermediate filaments are tension-bearing elements that help to maintain cell shape and rigidity. FPs contain longitudinal and arciform actin microfilaments and a thin cortex of actin filaments that run cortically and contiguously to link adjacent processes. Neighboring FPs are connected by a contractile apparatus consisting of F-actin, myosin II, β-actinin-4 and synaptopodin[6,7].
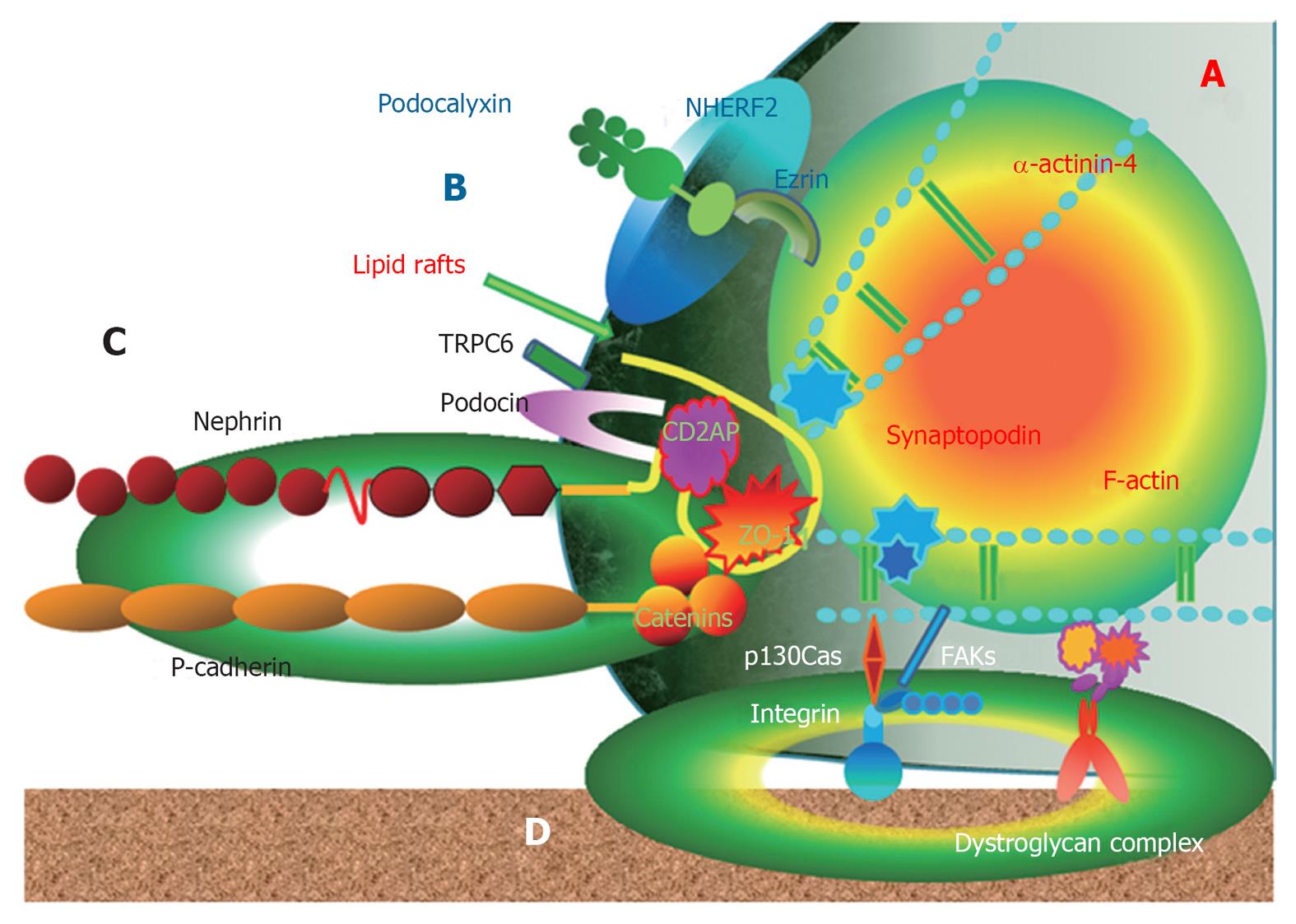
The FPs are anchored to the GBM via 1 integrin and dystroglycans and link the apical proteins, such as podocalyxin[1-6]. These anchoring molecules connect to the actin cytoskeleton via the intracellular macromolecular complex of the focal adhesion kinase (FAK) and molecules, including paxillin, vinculin, p130Cas and actinin[1-6]. Therefore, podocyte FPs represent a major center of podocyte function and are defined by the actin cytoskeleton and three membrane domains of podocytes: the apical membrane domain, the SD protein complex and the basal membrane domain[8,9]. The submembranous regions of all three membrane domains are connected to the FP actin cytoskeleton. Hence, the actin cytoskeleton determines the structural maintenance of the glomerular filtration units with three membrane domains[6-9] (Figure 1).
The proteinuric condition demonstrates stereotypical ultrastructural changes in the podocytes with retraction and effacement of the highly specialized interdigitating FP, regardless of the underlying diagnosis[6-10]. On the basis of recent progress in the molecular structural pathology of podocytes, four major causes can lead to FP effacement and proteinuria regarding the three membrane domains and cytoskeleton: (1) interference with the SD complex and its lipid rafts; (2) interference with the GBM or the podocyte-GBM interaction; (3) interference with the actin cytoskeleton and its associated proteins, including β-actinin-4 and synaptopodin; and (4) interference with the negative apical membrane domain of podocytes[8-10]. Interference with any of the three FP membrane domains leads to changes of the actin cytoskeleton from parallel contractile bundles into a dense network with FP effacement and obliteration of the filtration slits; therefore, FP effacement requires the active reorganization of actin filaments. As a reverse pathological pathway, any alterations in the cytoskeleton could also lead to changes in the structure and function of podocytes, resulting in proteinuria[6-10].
Lipid rafts are specialized microdomains of the plasma membrane enriched in cholesterol, glycosphingolipids and glycosylphosphatidylinositol-anchored proteins[11,12]. The structure of lipid rafts is dynamic, resulting in an ever-changing content of both lipids and proteins. By compartmentalizing cell membranes, they recruit and cluster membrane proteins in a selective and dynamic fashion. Hereby, they provide molecular frameworks for numerous cell biological processes, such as exocytosis and endocytosis, cell adhesion and signal transduction events[12]. The heterogeneous and dynamic makeup of these lipid raft domains contributes to the large number of signals capable of being transduced from the outer membrane of the cell to cellular organelles of the cytoplasm or the nucleus of the cell, through the lipid rafts. In many cases, the function of proteins depends greatly on their association with lipid rafts[11-13].
In a podocyte, lipid raft microdomains are critical for the dynamic functional organization of the SD[1] (Figure 1). Nephrin associates with lipid rafts and co-immunoprecipitates with a podocyte-specific 9-O-acetylated ganglioside and the in vivo injection of an antibody against this ganglioside causes morphological changes of the filtration slits, resembling FP effacement[14]. In this model, nephrin translocated to the apical pole of the narrowed filtration slits and underwent tyrosine phosphorylation[14]. Tyrosine phosphorylation of nephrin in SD is regulated by the Src family kinase Fyn, which was supported by the result that the genetic inactivation of the Src family kinase Fyn and Lyn caused proteinuria in mice[15]. Nephrin tyrosine phosphorylation also controls podocyte cell morphology through the Nck adaptor proteins in lipid rafts, associated with activated nephrin and nephrin-directed actin polymerization[16,17] (Figure 2). Podocin, associated with nephrin in lipid rafts, also binds and regulates the transient receptor potential channel TRPC6, mediating pressure sensation in podocytes[18]. Therefore, the nephrin-podocin protein complex in lipid rafts is an important structure, not only as a structural component of the SD structure, but also as an active signaling nexus modulating the structural and functional characteristics in podocytes[19].
Adaptor proteins are noncatalytic polypeptides that contain one or more protein interaction modules that mediate protein interactions[20]. Most adaptor proteins are localized at or near lipid rafts in podocytes and their interactions are regulated through phosphorylation and dephosphorylation of crucial protein and lipid substrates[19,21]. The adaptor proteins in podocytes are described as follows.
CD2AP was originally regarded as an adaptor protein interacting with the cytoplasmic domain of CD2 receptor in T cells and natural killer cells. CD2AP facilitates T cell adhesion to antigen-presenting cells by enhancing CD2 clustering and organizing the cytoskeleton around the interaction site needed for the polarization of T-cells[22]. In kidneys, CD2AP expresses predominantly in podocytes. CD2AP is expressed primarily in podocytes at the cytoplasmic face of the SD and lipid rafts and serves as an adaptor anchoring nephrin and podocin to actin filaments of podocyte cytoskeleton[23-25]. CD2AP and nephrin are tightly associated with podocin, forming nephrin-podocin-CD2AP complexes, and embedded in lipid rafts, where podocin interacts through its C-terminal end with CD2AP and nephrin[24,25]. CD2AP also serves as a platform transducing signals inward and/or outward[23-26]. CD2AP is closely localized with other podocyte proteins, such as p130Cas[26], actinin-4[26] and synaptopodin[27], and might interact with them functionally and structurally.
Early studies of mice lacking CD2AP, which develop progressive glomerulosclerosis and die of massive proteinuria at the age of 6-7 wk, have suggested the pivotal role of CD2AP in maintaining the structural integrity of the glomerular filtration barrier[27,28].
ZO-1 is a peripheral membrane protein associated with the cytoplasmic surface of the tight junction in all epithelial and endothelial cell types studied[29]. It is also expressed on the cytoplasmic surface of podocyte FPs at the insertion of the SD[3-5,30] and is linked to the actin cytoskeleton on the other side[3-5,31]. Therefore, ZO-1 protein as a component of the SD plays a pivotal role in maintaining the glomerular permeability by connecting the SD structure and actin cytoskeleton.
ZO-1 has been reported in several studies as one of the SD proteins that may be associated with the development of proteinuria. In experimental nephrosis, ZO-1 became discontinuous and concentrated along both the newly formed occluding-type junctions and the remaining SD in puromycin aminonucleoside (PAN) or protamine sulfate-treated rats[32]. MAb 5-1-6 against nephrin decreased the immunoreactivity of ZO-1 in glomeruli with heavy proteinuria, but did not induce changes in the structural integrity of the SD; therefore, suggesting that mAb 5-1-6 alters the molecular composition of the SD and thereby affects the glomerular permeability barrier[33]. Liu et al[34] also found that disruption of the NEPH1-nephrin interaction in vivo by injecting combinations of individual subnephritogenic doses of anti-NEPH1 and anti-nephrin resulted in complement- and leukocyte-independent proteinuria with preserved FPs. This disruption of the NEPH1-nephrin interaction reduces NEPH1 and nephrin protein expression modestly and also reduces ZO-1 protein expression dramatically via the interaction of ZO-1 PDZ domains with the cytoplasmic tail of NEPH1. In MWF rats, an animal model of spontaneous proteinuria, proteinuria developed without significant changes in the permeability of the GBM to water and albumin, or in the ultrastructure of the podocyte FPs, but was associated with an important alteration in the distribution of ZO-1 at the glomerular level[35]. In summary, in the results of in vivo experimental nephrosis, ZO-1 redistribution was associated with changes in the foot process ultrastructure and apical displacement of the glomerular SD.
In diabetic conditions, hyperglycemia in diabetic animals and high glucose on cultured glomerular epithelial cells induced a decrease in the intensity of ZO-1 staining and redistribution of ZO-1 from the membrane to the cytoplasm[36]. We presented that ZO-1 is uniformly distributed along the outer area of the cytoplasm of cultured podocytes in normal condition, whereas this pattern is markedly altered to be discontinuous in a high glucose and advanced glycosylation end products-treated condition with inner redistribution of the ZO-1[37]. Therefore, a disruption of the cell-to-cell size-selective SD barrier, including ZO-1, is a notable finding in podocyte hyperpermeable conditions.
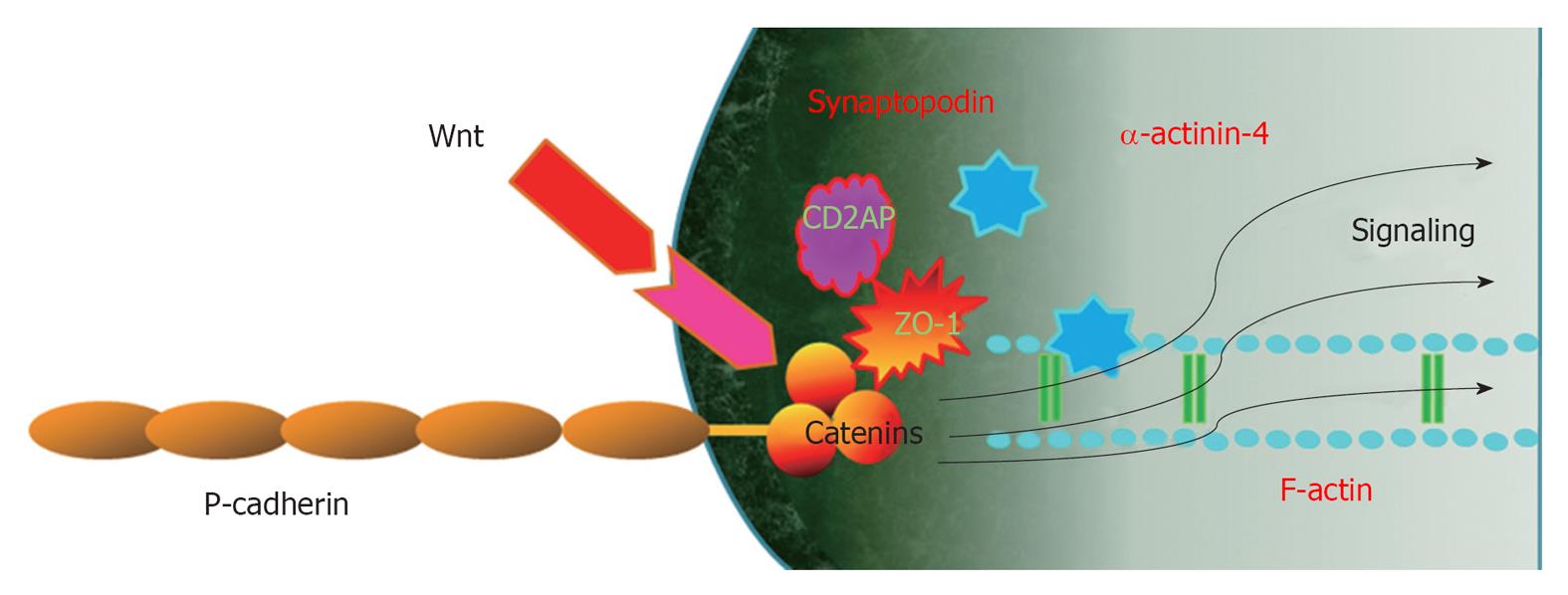
β-catenin is known as a “dual-function” protein in epithelial cells and its function is largely determined by its subcellular localization[38]. β-catenin in the plasma membrane is a central component of the cadherin/catenin adhesive complex that stabilizes cell adherens junctions to maintain epithelial integrity. β-catenin in the nucleus, however, acts as a key regulator of gene expression via binding to transcription factors such as T-cell factor and lymphoid enhancer-binding factor[39]. The canonical pathway regulating β-catenin involves glycogen synthase kinase-3β (GSK-3β)/Wnt signaling. β-catenin of podocytes also has dual functions depending on subcellular localization. In lipid rafts, β-catenin is a component of the P-cadherin/β-catenin complex that stabilizes cell adherens junctions to maintain podocyte SD integrity and links to cytoskeleton[1,2]. In the nucleus, however, β-catenin acts as a key regulator of gene expression via binding to transcription factors.
Studies on the changes of β-catenin of podocytes in diseases are limited to its activity and Wnt/β-catenin signaling. Wnt/β-catenin signaling is a critical player in the pathogenesis of podocyte dysfunction and albuminuria. Wnt/β-catenin is activated in the podocytes of mouse and human kidneys with primary glomerular disorders, such as adriamycin-induced nephropathy, diabetic nephropathy and focal segmental glomerulosclerosis[40,41]. Ectopic expression of Wnt1 gene in mice exacerbates podocyte injury and albuminuria, whereas blockade of Wnt signaling with its antagonist DKK1 ameliorates podocyte lesions[42]. Transforming growth factor-1 (TGF-1) is sufficient to induce podocyte injury, including epithelial-to-mesenchymal transition, and proteinuria, which is accompanied by Wnt1 induction, β-catenin activation and induction of numerous Wnt target genes in the glomeruli[43,44]. Inhibition of Wnt signaling by its antagonist ameliorates TGF-β1-triggered podocyte injury in vivo, suggesting a crucial role for the canonical Wnt/β-catenin signaling in mediating podocyte injury[44]. Therefore, gain or loss of function experiments in whole animal at Wnt/β-catenin levels suggests that the hyperactive Wnt/β-catenin signaling is a crucial mediator in inducing podocyte injury.
The Nck proteins (Nck1 and Nck2) are composed of an SH2 domain, which can interact with phosphotyrosines, and of SH3 domains, which can recruit several other proteins involved in the regulation of actin assembly. Nephrin associates with an adaptor protein, Nck, via its tyrosine-phosphorylated binding sites. This interaction is mediated by the SH2 domain of Nck and several tyrosine-phosphorylated binding sites in nephrin, and the phosphorylation of specific binding sites in nephrin is dependent on Fyn-kinase[16,45]. The mice lacking both the Nck1 and Nck2 genes from the podocytes developed nephrotic range proteinuria. In addition, histological and electron microscopic examination revealed fusion of the cellular projections and lesions similar to those in human end-stage renal disease[45].
p130Cas is a protein of the Crk-associated substrate family that might serve as an ubiquitous docking protein in various tissues and cellular structures, including podocytes, for actin cytoskeleton-dependent signaling networks. The interaction of p130Cas with other adjacent proteins in normal and pathological cells modulates cell migration, survival and proliferation[46,47]. In podocytes, p130Cas localizes diffusely to the cytoplasm with accumulation at the ends of F-actin stress fibers in FPs, where FAKs connect docking proteins, including integrin and p130Cas, to the GBM and CD2AP and p130Cas to the SD insertion site[26]. Therefore, p130Cas protein also plays an important role in maintaining the glomerular permeability by connecting the podocyte actin cytoskeleton to GBM and SD.
There are very limited reports on the change of p130Cas in pathological conditions until now. The immunofluorescent staining of p130Cas increased around the glomerular capillary loop of human membranous nephropathy; however, not of minimal change disease[48]. They suggested that the increased p130Cas might be a result of tyrosine phosphorylation of constituent proteins. Although both diseases are podocyte diseases, the pathophysiological mechanisms leading to membranous nephropathy and minimal change disease are different. Membranous nephropathy is caused by an accumulation of immune deposits on the outer aspect of the GBM. However, minimal change disease is characterized by podocyte phenotypical changes caused by plasma permeability factors[49]. In animal models with immune-mediated glomerular diseases, increased tyrosine phosphorylation within focal adhesion proteins, increased Pyk2 and FAK activation have been reported[50-52]. Therefore, the expression of podocyte p130Cas could be different, according to the pathophysiological mechanisms leading to podocytopathy.
Adaptor proteins in podocytes play important roles, not only as a structural component of the podocyte structure, but also as an active signaling platform.
The FPs contain actin-based highly ordered bundles that run parallel to the longitudinal axis of FPs[6,7]. The cytoskeleton is connected to three plasma membrane domains (basal, lateral and apical) of FPs via several linker adaptor proteins[1-4].
SD containing nephrin, P-cadherin, NEPH1, FAT etc. serves as a size-selective barrier and is linked to the actin-based cytoskeleton by adaptor proteins, including CD2AP, ZO-1, β-catenin, podocin and Nck[1-4,16]. CD2AP binds directly to nephrin and actin and serves as a direct link between the SD and the actin cytoskeleton. This link is essential for functional renal filtration, as mice lacking CD2AP develop FP effacement and progressive glomerulosclerosis and die of massive proteinuria[27].
Nck in podocytes has been shown to interact with tyrosine phosphorylated nephrin[16,45]. This interaction is required for the development of the normal filtration barrier, as mice lacking both Nck proteins in podocytes develop FP effacement and massive proteinuria[45]. Also, Nck proteins are needed in the maintenance of the mature filtration barrier, as inactivation of Nck proteins in adult mouse podocytes result in proteinuria and FP effacement[53]. Hence, Nck-nephrin interaction is required for nephrin-dependent actin reorganization. Taken together, both CD2AP and Nck proteins are crucial for linking the nephrin and podocin of SD to the actin cytoskeleton. These interactions mediate the actin polymerization and the cytoskeletal reorganization in FP that is required for the normal functional filtration barrier.
NEPH1 with five extracellular IgG-like motifs is located at the SD and structurally related to nephrin[4]. Phosphorylation of NEPH1 augmented actin polymerization is in response to nephrin phosphorylation, via Nck proteins and recruitment of Grb2[54]. NEPH1-deficient mice show FP effacement and die perinatally due to massive proteinuria[55]. Disruption of NEPH1-nephrin interaction in vivo by injecting combinations of individual subnephritogenic doses of anti-NEPH1 and anti-nephrin results in complement- and leukocyte-independent proteinuria with preserved FPs[34]. This disruption modestly reduces NEPH1 and nephrin protein expression in podocytes and dramatically reduces ZO-1 protein expression via the interaction of ZO-1 PDZ domains with the cytoplasmic tail of NEPH1[34]. Renal ischemia by ATP depletion induced rapid loss of NEPH1 and ZO-1 binding and redistribution of NEPH1 and ZO-1 proteins from the cell membrane to the cytoplasm and recovery resulted in increased NEPH1 tyrosine phosphorylation mediated by Fyn and restored NEPH1 and ZO-1 binding and their localization at the cell membrane, suggesting a critical role for NEPH1 tyrosine phosphorylation in reorganizing the NEPH1-ZO-1 complex[56]. Taken together, both CD2AP and Nck proteins are crucial in linking the SD proteins, including nephrin, podocin and NEPH1 to actin cytoskeleton.
Another important SD complex is the P-cadherin/β-catenin complex. Unlike nephrin and NEPH1, P-cadherin-deficient mice do not appear to have a significant renal phenotype[57], suggesting that P cadherin may not be involved critically in the development of a normal glomerular capillary loop. Another study was unable to show an in vivo or in vitro association of P-cadherin with nephrin or NEPH1[3]. Members of the catenin family bind the cytoplasmic segment of P-cadherin in the glomerular capillary loop[1-3,58]. Therefore, β-catenin in the lipid rafts of podocytes is a component of the P-cadherin/β-catenin complex that stabilizes cell adherens junctions to maintain podocyte SD integrity and links to cytoskeleton[1,2]. P-cadherin also related to ZO-1 structurally and functionally[30]. Both the nephrin-podocin-CD2AP and P-cadherin/β-catenin complex are schematically drawn in Figure 2.
The podocyte, as all epithelial cells, is attached to the underlying GBM through transmembrane cell receptors, such as integrins, tetraspanins and dystroglycans. p130Cas localizes diffusely to the cytoplasm with accumulation at the ends of F-actin stress fibers in FPs and connects cytoskeleton and FAKs to integrin and then GBM[26]. p130Cas also colocalizes with CD2AP in the SD insertion site[26]. Recently, we observed that the fluorescences of the p130Cas protein were internalized and became granular and its protein and mRNA expression levels of p130Cas were suppressed by PAN, which were reversed by antioxidants[59]. Therefore, p130Cas protein also plays an important role in maintaining the glomerular permeability by connecting the podocyte actin cytoskeleton to GBM and SD.
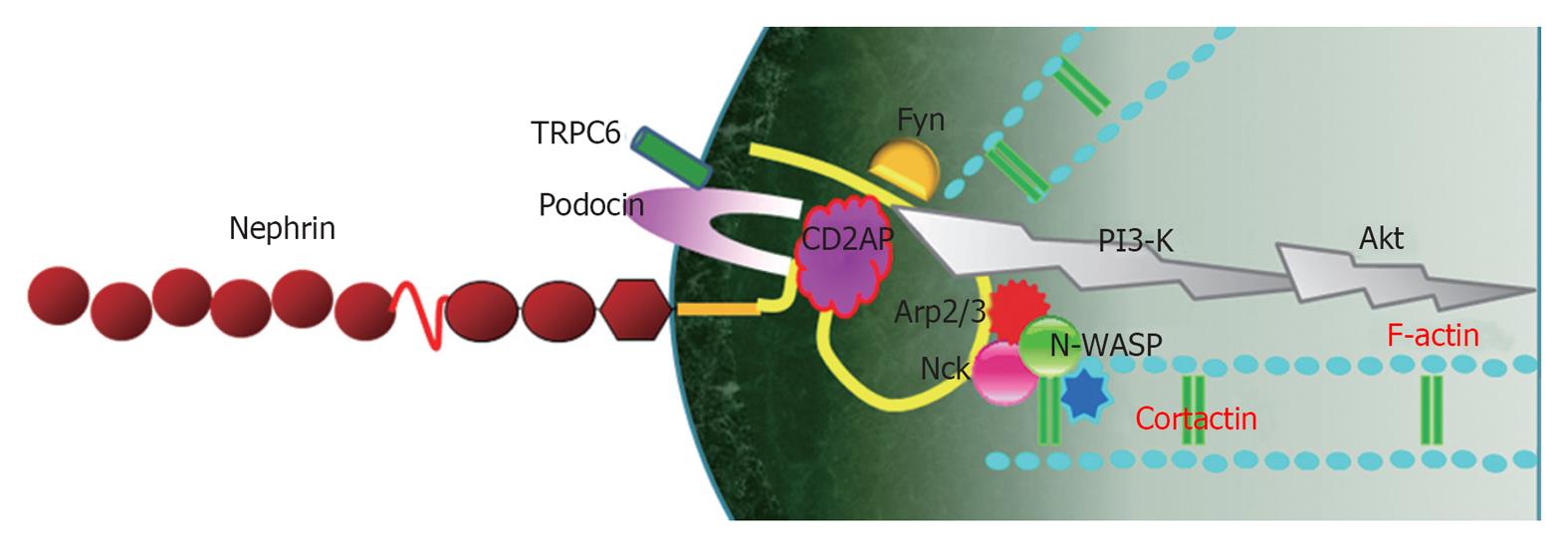
Nephrin-Nck-neuronal Wiskott-Aldrich syndrome protein complex: Nephrin molecules from adjacent FPs interact with each other in an antiparallel, homophilic manner, serving not only as a structural backbone of the SD, but also as a component of the SD-lipid rafts protein complex, which transmits signals into the cells[21,60]. Therefore, nephrin acts as a transducer of the extracellular signals from the SD to the intracellular actin cytoskeleton[6,7]. The cytoplasmic tail of nephrin binds to adaptor proteins, such as CD2AP, Nck2 and densin[61]. These adaptor proteins interact directly with actin or indirectly through actin-binding proteins, such as β-actinin-4, synaptopodin, cofilin, fimbrin etc.[21,60,61].
The cytoplasmic domain of nephrin contains six conserved tyrosine residues, which can be phosphorylated by members of the Src-kinase family during renal development and under pathological conditions[14,16]. Tyrosine phosphorylation of nephrin is dependent on its interaction with a number of nephrin-binding proteins, which stabilize nephrin at the SD and coordinate nephrin signaling[60]. Following nephrin phosphorylation through Fyn[62], the SH2 domain of Nck binds to phospho-nephrin and the SH3 domains of Nck bind to neuronal Wiskott-Aldrich syndrome protein (N-WASP)[16,45]. N-WASP, in turn, activates the Arp2/3 complex and cortactin, thereby linking the nephrin-Nck complex to the podocyte actin cytoskeleton[6,7]. Nck and its associated actin cytoskeleton regulatory proteins are recruited to the phosphorylated nephrin when rapid actin polymerization and cytoskeletal reorganization is required during development and injury repair[16]. However, in a steady state, nephrin-Nck interactions might be low and nephrin-CD2AP interactions predominate[21,63] (Figure 2).
Nephrin-CD2AP-phosphoinositide 3-kinase/Akt complex: Another signaling pathway from extracellular SD to intracellular cytoskeleton through nephrin is via the nephrin-podocin-CD2AP complex[64]. CD2AP and nephrin interact with a subunit of phosphoinositide 3-kinase (PI3-K) and subsequently stimulate PI3-K-dependent activation of the intracellular Akt kinase pathway, which is necessary for the regulation of actin dynamics and the cell survival[7,64]. PI3-K and its downstream mediators Akt play a central role in a diverse range of cellular responses, including cell growth, survival, proteolysis and malignant transformation[65,66].
Although the importance of this signaling in podocytes is not fully understood, one target substrate of nephrin/CD2AP-induced phosphorylation is Bad, a proapoptotic protein of the Bcl-2 family; phosphorylated Bad is inactivated and apoptosis does not occur[64]. Activation of the PI3-K/Akt pathway by nephrin is protected against detachment-induced apoptosis of cultured murine podocytes[64]. The antiapoptotic effect of Akt on podocyte apoptosis is further supported by the observation that a failure to phosphorylate Akt causes apoptosis of podocytes in db/db mice[67], in oxidized LDL-induced podocyte injury[68] and in CD2AP-deficient mice[69]. It has been shown that loss of CD2AP leads to increased expression of TGF-β1 in podocytes and apoptosis in CD2AP-/- mice[69,70], and CD2AP was required for the early activation of anti-apoptotic PI3-K/Akt and extracellular signal-regulated kinase 1/2 by TGF-β[69]. Similarly, in TGF-β1 transgenic mice with heterozygous CD2AP, CD2AP heterozygosity increased both podocyte apoptosis and proteinuria, suggesting that noncanonical CD2AP/PI3-K/Akt signaling modules mediate anti-apoptosis of podocytes[71]. In an in vitro albumin overload model, albumin overload and accumulation in podocytes induced endoplasmic reticulum (ER) stress and apoptosis and downregulated the expression of CD2AP. In addition, downregulation of CD2AP expression by CD2AP siRNA transfection deteriorated the changes induced by albumin overload. On the other hand, transfection of CD2AP eukaryotic expression vector into podocytes increased CD2AP expression and inhibited podocyte ER stress and apoptosis. Therefore, CD2AP plays a preventive role in albumin overload-induced ER stress and apoptosis in podocytes[72].
Another important role of the nephrin-CD2AP-PI3-K/Akt complex is the regulation of the actin cytoskeleton of podocytes[6]. Stable transfection of rat nephrin in the podocytes with podocin led to nephrin tyrosine phosphorylation, PI3-K-dependent phosphorylation of Akt, increased Rac1 activity and an altered actin cytoskeleton with decreased stress fibers and increased lamellipodia. On the other hand, in the rat model of PAN nephrosis, nephrin tyrosine phosphorylation, nephrin-PI3-K association and glomerular Akt phosphorylation were all decreased[73]. CD2AP-/- mice develop nephrotic syndrome shortly after birth and die at around 6-7 wk of age from renal failure. Electron microscopy reveals extensive foot process effacements and this podocyte injury might lead to these secondary mesangial cell abnormalities. In CD2AP knock-down podocytes by siRNA, cell adhesion and spreading ability decreased with disordered distributions of F-actin and nephrin expression and phosphorylation were also reduced[74].
The interaction of nephrin with PI3-K results in the dephosphorylation and activation of cofilin, a member of the ADF/cofilin family[75]. Cofilin is an actin-binding protein that binds to F-actin filaments and causes depolymerization at the minus end of filaments, thereby preventing their reassembly, and disassembles actin filaments; therefore, cofilin is essential for the remodeling and elongation of actin filaments[76]. It is thought that regulation of cofilin activity by nephrin-PI3-K signaling is essential for the maintenance of the normal podocyte cytoskeleton and in response to injury[7,75]. Therefore, the nephrin-CD2AP-PI3-K/Akt complex is deeply involved in SD-mediated actin reorganization in podocytes (Figure 2). Recently, we reported that diabetic conditions induced the re-localization and concentration of CD2AP at internal cytoplasmic and perinuclear areas of podocytes and decreased the CD2AP protein amount and its mRNA expression, which were prevented by LY294002, a PI3-K inhibitor[77]. Therefore, CD2AP/PI3-K/Akt signaling might also be important for the maintenance of the podocyte SD integrity in response to diabetic podocyte injury.
PI3-K/Akt signaling is also related to the changes of ZO-1 in mammary epithelial cells[78] and podocytes[37]. We found that diabetic conditions induced the activation of PKB/Akt via PI3-K and then the downstream pathways change the metabolism and transport of SD ZO-1 protein and cell integrity, causing hyperpermeability subsequently[37].
P-Cadherin-β-catenin-Wnt signaling:β-catenin of podocytes has been identified as a cytoplasmic anchorage protein for P-cadherin, forming the P-cadherin/β-catenin complex[5]. This complex stabilizes functional adherens junctions of podocyte SD and links to the actin cytoskeleton and β-catenin also participates in signaling pathways as a Wnt/β-catenin signaling which acts as a key regulator of gene expression via binding to transcription factors.
In the absence of the Wnt ligand, β-catenin is targeted for degradation via phosphorylation of its serine (Ser-33/34/45) and threonine (Thr-41) sites by GSK-3β[79]. Upon binding to their receptors/coreceptors, Wnt proteins induce a series of downstream signaling events, including inhibition of GSK-3β, resulting in β-catenin dephosphorylation and stabilization. This allows β-catenin to translocate into the nuclei, wherein it binds to T cell factor/lymphoid enhancer-binding factor to stimulate the transcription of Wnt target genes[80]. On the basis of this canonical pathway of Wnt signaling, it is conceivable that either inhibiting Wnt expression or repressing β-catenin transcriptional activity could be an effective way to control the Wnt/β-catenin signaling.
Wnt/β-catenin signaling is an evolutionarily conserved cellular signaling system that plays an essential role in a diverse array of biological processes, such as differentiation/organogenesis, cell adhesion, survival/apoptosis, tissue homeostasis and pathogenesis of many human diseases[79]. In podocytes, Wnt/β-catenin signaling plays an important role in cell adhesion, differentiation and survival/apoptosis[81] (Figure 3). The Wnt/β-catenin pathway appears to interact with integrin and FAK signaling pathways in podocytes, as in intestinal epithelium[82]. Therefore, the overall effect of Wnt/β-catenin activation is related to podocyte detachment and the development of albuminuria. The activation of the Wnt/β-catenin pathway is observed following apoptosis or injury and could contribute to organ regeneration. The Wnt/β-catenin pathway is regarded as a strong pro-survival signal; therefore, Wnt pathway activation might represent a survival signal in pathological podocytes. However, there is no direct evidence to date to conclusively demonstrate that activation of the Wnt/β-catenin pathway in the podocyte protects against apoptosis.
In diabetic nephropathy, increased activation of the pathway might occur to promote podocyte survival but it also leads to cell detachment and podocyte loss. Down-regulation of the Wnt/β-catenin pathway in podocytes might be important for terminal differentiation; however, it enhances apoptosis susceptibility[82]. Therefore, balanced Wnt/β-catenin signaling is critical for podocyte biology in normal and injury conditions.
The adaptor proteins, such as CD2AP, ZO-1, β-catenin, Nck, p130Cas, located at intracellular SD insertion area of podocytes have, not only an important structural role, but also a signaling role from SD to the actin cytoskeleton.
P-Reviewer Li ST S- Editor Song XX L- Editor Roemmele A E- Editor Zheng XM
| 1. | Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005-3015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 2. | Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1-8. [PubMed] [Cited in This Article: ] |
| 4. | Patrakka J, Tryggvason K. Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun. 2010;396:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253-307. [PubMed] [Cited in This Article: ] |
| 6. | Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 412] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 7. | Welsh GI, Saleem MA. The podocyte cytoskeleton--key to a functioning glomerulus in health and disease. Nat Rev Nephrol. 2012;8:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Oh J, Reiser J, Mundel P. Dynamic (re)organization of the podocyte actin cytoskeleton in the nephrotic syndrome. Pediatr Nephrol. 2004;19:130-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Smoyer WE, Mundel P. Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med (Berl). 1998;76:172-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31-39. [PubMed] [Cited in This Article: ] |
| 12. | Cherukuri A, Dykstra M, Pierce SK. Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity. 2001;14:657-660. [PubMed] [Cited in This Article: ] |
| 13. | George KS, Wu S. Lipid raft: A floating island of death or survival. Toxicol Appl Pharmacol. 2012;259:311-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, Holthöfer H. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol. 2001;159:1069-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr Biol. 2001;11:34-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346-1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int. 2008;73:926-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA. 2006;103:17079-17086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Lehtonen S. Connecting the interpodocyte slit diaphragm and actin dynamics: Emerging role for the nephrin signaling complex. Kidney Int. 2008;73:903-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Pawson T. Protein modules and signalling networks. Nature. 1995;373:573-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1803] [Cited by in F6Publishing: 1743] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 21. | Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol. 2004;15:1382-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 22. | Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 561] [Cited by in F6Publishing: 574] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 23. | Li C, Ruotsalainen V, Tryggvason K, Shaw AS, Miner JH. CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol Renal Physiol. 2000;279:F785-F792. [PubMed] [Cited in This Article: ] |
| 24. | Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303-2308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 412] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Welsch T, Endlich N, Kriz W, Endlich K. CD2AP and p130Cas localize to different F-actin structures in podocytes. Am J Physiol Renal Physiol. 2001;281:F769-F777. [PubMed] [Cited in This Article: ] |
| 27. | Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 28. | Shaw AS, Miner JH. CD2-associated protein and the kidney. Curr Opin Nephrol Hypertens. 2001;10:19-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1148] [Cited by in F6Publishing: 1192] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 30. | Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 247] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 524] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 32. | Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol. 1992;141:805-816. [PubMed] [Cited in This Article: ] |
| 33. | Kawachi H, Kurihara H, Topham PS, Brown D, Shia MA, Orikasa M, Shimizu F, Salant DJ. Slit diaphragm-reactive nephritogenic MAb 5-1-6 alters expression of ZO-1 in rat podocytes. Am J Physiol. 1997;273:F984-F993. [PubMed] [Cited in This Article: ] |
| 34. | Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209-221. [PubMed] [Cited in This Article: ] |
| 35. | Macconi D, Ghilardi M, Bonassi ME, Mohamed EI, Abbate M, Colombi F, Remuzzi G, Remuzzi A. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000;11:477-489. [PubMed] [Cited in This Article: ] |
| 36. | Rincon-Choles H, Vasylyeva TL, Pergola PE, Bhandari B, Bhandari K, Zhang JH, Wang W, Gorin Y, Barnes JL, Abboud HE. ZO-1 expression and phosphorylation in diabetic nephropathy. Diabetes. 2006;55:894-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Ha TS. High-glucose and advanced glycosylation end products increased podocyte permeability via PI3-K/Akt signaling. J Mol Med (Berl). 2010;88:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Peng J, Dong Z. Role changes of β-catenin in kidney injury and repair. Kidney Int. 2012;82:509-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 359] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 40. | Heikkilä E, Juhila J, Lassila M, Messing M, Perälä N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H. beta-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant. 2010;25:2437-2446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 42. | Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997-2008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 43. | Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 44. | Wang D, Dai C, Li Y, Liu Y. Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int. 2011;80:1159-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 46. | O’Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111-119. [PubMed] [Cited in This Article: ] |
| 47. | Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 48. | Bains R, Furness PN, Critchley DR. A quantitative immunofluorescence study of glomerular cell adhesion proteins in proteinuric states. J Pathol. 1997;183:272-280. [PubMed] [Cited in This Article: ] |
| 49. | Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 512] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 50. | Takagi C, Ueki K, Ikeuchi H, Kuroiwa T, Kaneko Y, Tsukada Y, Maezawa A, Mitaka T, Sasaki T, Nojima Y. Increased expression of cell adhesion kinase beta in human and rat crescentic glomerulonephritis. Am J Kidney Dis. 2002;39:174-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Morino N, Matsumoto T, Ueki K, Mimura T, Hamasaki K, Kanda H, Naruse T, Yazaki Y, Nojima Y. Glomerular overexpression and increased tyrosine phosphorylation of focal adhesion kinase p125FAK in lupus-prone MRL/MP-lpr/lpr mice. Immunology. 1999;97:634-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma M, Yu Z, Ardito T, Czyzyk J, Diggs L. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol. 2010;21:1145-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 53. | Jones N, New LA, Fortino MA, Eremina V, Ruston J, Blasutig IM, Aoudjit L, Zou Y, Liu X, Yu GL. Nck proteins maintain the adult glomerular filtration barrier. J Am Soc Nephrol. 2009;20:1533-1543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27:8698-8712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829-4836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 342] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 56. | Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB. Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem. 2008;283:35579-35589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 58. | Piepenhagen PA, Nelson WJ. Differential expression of cell-cell and cell-substratum adhesion proteins along the kidney nephron. Am J Physiol. 1995;269:C1433-C1449. [PubMed] [Cited in This Article: ] |
| 59. | Ha TS, Choi JY, Park HY. Puromycin aminonucleoside modulates p130Cas of podocytes. Korean J Pediatr. 2012;55:371-376. [PubMed] [Cited in This Article: ] |
| 60. | Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens. 2005;14:211-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 61. | Patrakka J, Tryggvason K. Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol Med. 2007;13:396-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278:20716-20723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Putaala H, Sainio K, Sariola H, Tryggvason K. Primary structure of mouse and rat nephrin cDNA and structure and expression of the mouse gene. J Am Soc Nephrol. 2000;11:991-1001. [PubMed] [Cited in This Article: ] |
| 64. | Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917-4928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 65. | Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1-13. [PubMed] [Cited in This Article: ] |
| 66. | Foster FM, Traer CJ, Abraham SM, Fry MJ. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116:3037-3040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 67. | Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936-1947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 69. | Schiffer M, Mundel P, Shaw AS, Böttinger EP. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem. 2004;279:37004-37012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 70. | Woroniecki RP, Schiffer M, Shaw AS, Kaskel FJ, Bottinger EP. Glomerular expression of transforming growth factor-beta (TGF-beta) isoforms in mice lacking CD2-associated protein. Pediatr Nephrol. 2006;21:333-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Xavier S, Niranjan T, Krick S, Zhang T, Ju W, Shaw AS, Schiffer M, Böttinger EP. TbetaRI independently activates Smad- and CD2AP-dependent pathways in podocytes. J Am Soc Nephrol. 2009;20:2127-2137. [PubMed] [Cited in This Article: ] |
| 72. | He F, Chen S, Wang H, Shao N, Tian X, Jiang H, Liu J, Zhu Z, Meng X, Zhang C. Regulation of CD2-associated protein influences podocyte endoplasmic reticulum stress-mediated apoptosis induced by albumin overload. Gene. 2011;484:18-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 74. | Zhang C, Jiang HJ, Chang Y, Fang Z, Sun XF, Liu JS, Deng AG, Zhu ZH. Downregulation of CD2-associated protein impaired the physiological functions of podocytes. Cell Biol Int. 2009;33:632-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, Mizuno K, Gurniak C, Witke W, Holzman LB. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem. 2010;285:22676-22688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J Cell Biol. 1997;138:771-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 572] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 77. | Ha TS, Hong EJ, Han GD. Diabetic conditions downregulate the expression of CD2AP in podocytes via PI3-K/Akt signaling. Diabetes Metab Res Rev. 2013;In press. [Cited in This Article: ] |
| 78. | Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803-36810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 770] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 79. | Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1377] [Cited by in F6Publishing: 1412] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 80. | Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 745] [Cited by in F6Publishing: 826] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 81. | Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem. 2011;286:26003-26015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 82. | Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |