Published online Mar 25, 2022. doi: 10.5527/wjn.v11.i2.73
Peer-review started: December 7, 2021
First decision: January 25, 2022
Revised: January 31, 2022
Accepted: March 23, 2022
Article in press: March 23, 2022
Published online: March 25, 2022
Nephritic syndrome (NiS) is a major indicator of serious renal diseases necessitating kidney biopsies for histopathological evaluations, but due to the lack of comprehensive reviews in the literature, the current understanding of the syndrome and its significance is limited.
To collect all the evidence retrievable from the literature on the diagnoses made on the renal biopsies performed for NiS as the indication to the procedure.
A literature search was conducted to find studies reporting final diagnoses on renal biopsies in NiS patients. Data were pooled and analyzed with stratifications on age and regions. Meta-analyzes were performed using Stata v.9.
Overall, 26414 NiS patients from the total number of 96738 kidney biopsy diagnoses reported by 47 studies from 23 countries from all continents (except sub-Saharan Africa) were found and analyzed. NiS was the indication for renal biopsy in 21% of the patient populations across the reviewed studies. Immunoglobulin A (IgA) nephropathy was the single most frequent diagnosis in these patients (approximately 38%) followed by lupus nephritis (approximately 8%) and Henoch Schönlein purpura (approximately 7%). IgA nephropathy was the most frequent diagnosis reported for the NiS patients from the East Asia, comprising half of all the cases, and least prevalent in South Asia. Considering the age subgroups, adult (vs pediatric or elderly) patients were by far the most likely age group to be diagnosed with the IgA nephropathy. A myriad of such regional and age disparities have been found and reported.
As the indication for renal biopsy, NiS represents a very distinctive epidemiology of final renal disease diagnoses compared to the other major syndromes.
Core Tip: Despite the extreme relevance of the renal biopsies in patients with different clinical syndromes and the final diagnoses that are being assigned to them, the current knowledge on the epidemiology of such diagnoses for nephritic syndrome is limited. This lack of understanding becomes more prominent when it comes to specific subpopulations, for example subgroups regarding age, ethnicity and global regions. This study tried to answer these questions, finding quite unprecedented, interesting, and clinically relevant findings.
- Citation: Taheri S. Renal biopsy reports in nephritic syndrome: Update. World J Nephrol 2022; 11(2): 73-85
- URL: https://www.wjgnet.com/2220-6124/full/v11/i2/73.htm
- DOI: https://dx.doi.org/10.5527/wjn.v11.i2.73
Renal disease is a major public health concern and a subject for considerable financial and mortality burden. However, different kidney diseases generally emerge with a limited spectrum of presentations most notably proteinuria and hematuria, different constellations of which comprise specific renal syndromes. These syndromes are not considered the final diagnosis of a specific renal disease, but rather they allude to specific renal diseases of different epidemiological magnitudes. In the approach to diagnose the culprit disorders, panels of experts have introduced definite indications for renal biopsies to be performed based on the presence or absence of these clinical syndromes.
Characterized by hematuria, elevated blood pressure, edema, and decrease in urine output, nephritic syndrome (NiS) is a major indicator of serious renal diseases necessitating kidney biopsies for histopathological evaluations. According to the published statistics for the year 2017, along with the nephrotic syndrome, NiS was reportedly the 9th leading cause of death in the United States[1], and extensive data from all around the world suggests consistent risk pattern for other global regions as well. Despite the invaluable data in the literature on the subject in general, scarcity of information exists on the estimated rates of the renal disease entities diagnosed upon analysis of renal biopsies for each renal syndrome. In two previous publications, the current author addressed the abovementioned issues for nephrotic syndrome, as well as subnephrotic proteinuria[2,3]. In the current study, NiS is the subject of the systematic review.
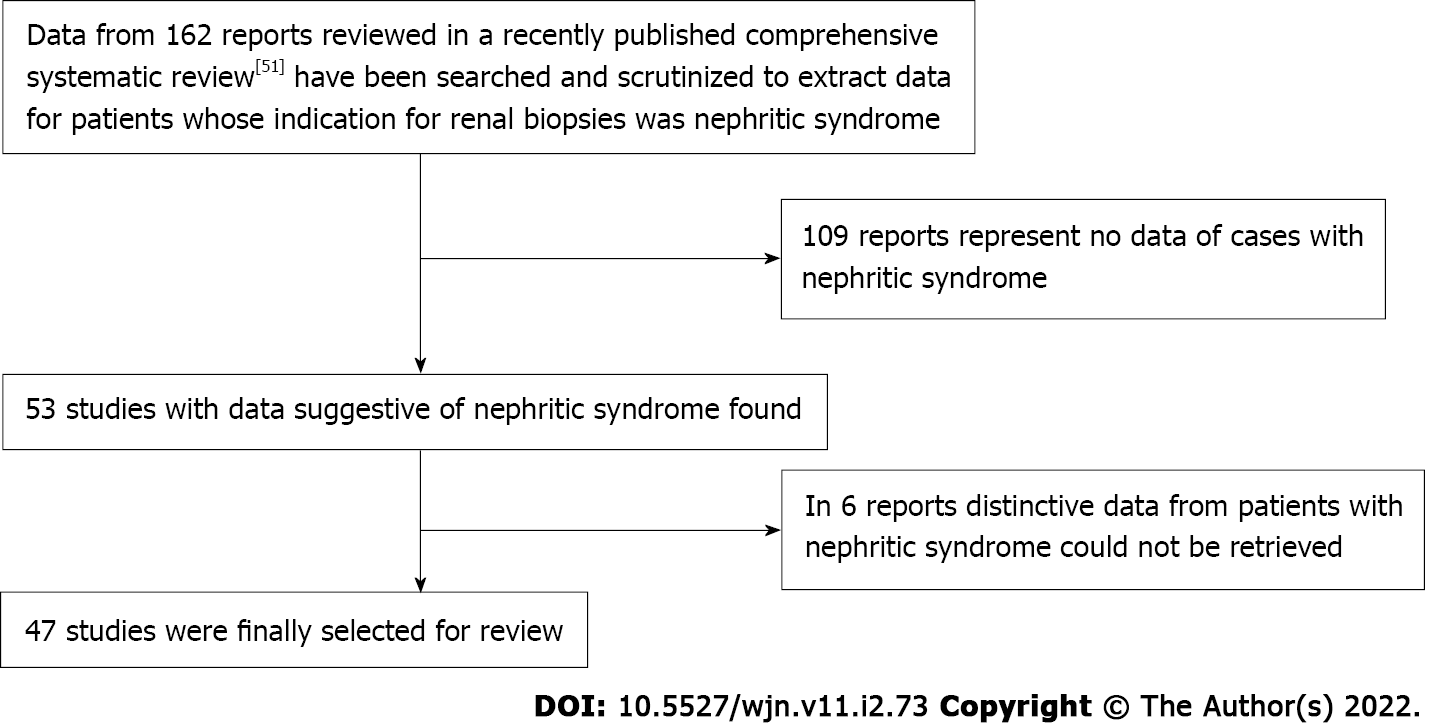
Figure 1 summarizes the study search and selection processes. This study aims to review the literature on the epidemiology of renal disease diagnoses made through investigating renal biopsy specimens from patients with NiS. One hundred and sixty-two reports were originally identified. After a preliminary review on the renal biopsy diagnoses (irrespective of their clinical syndromes), for studies whose data for NiS could be retrieved, 47 reports[4-50] were fully reviewed for this report. More detailed information on the methodology of this series of systematic reviews are published elsewhere, including two other reports on the epidemiology of nephrotic syndrome and subnephrotic proteinuria[2,3].
NiS was diagnosed when criteria for the NiS (hematuria, elevated blood pressure, decreased urine output, and edema) were fulfilled or the reports were clearly reporting either acute or chronic NiS, NiS (not otherwise specified), or NiS with nephrotic-range proteinuria (NiS-NS). Only definitive cases of NiS were included in the analysis while those with vague or equivocal data were excluded.
Renal disease diagnoses: Renal disease diagnoses included immunoglobulin A (IgA) nephropathy (Berger’s Disease), Henoch Schönlein purpura (HSP), Membranous glomerulonephritis (MGN), focal & segmental glomerulosclerosis (FSGS), lupus nephritis, mesangioproliferative glomerulonephritis (MesPGN), membranoproliferative glomerulonephritis (MPGN), amyloidosis, diabetic nephropathy, crescentric glomerulonephritis (CresGN), minimal change disease (MCD), tubulointerstitial diseases (TID), vascular nephropathy, nephroangiosclerosis (NAS), hereditary nephropathy, uspecific paraproteinemias (PPEs), and uspecific proliferative glomerulonephritis (PGN). Further specifications of the diagnoses have been published previously.
World regions: World regions were defined as follows: Middle East (including Egypt, Iran, Iraq, Jordan, Kingdom of Saudi Arabia, and Kuwait), Europe (including Belgium, Croatia, Cyprus, Czech R, Poland, Portugal, Romania, Serbia, and Turkey), Latin America (including Brazil and Colombia), East Asia (including China and Japan), South Asia (including India and Pakistan), and United States-Canada-Australia (USCA) (including United States & Australia).
Age groups: Age groups were defined as ‘pediatrics’, ‘adults’, ‘elderly’, and ‘general’. Pediatric group included patients 18 years of age or younger. Adults were classified for study populations older than 18 and younger than 65 years. However, some studies had inconsistent age categorizations. For example, in some studies, the lower limits of the range of patients’ ages was lower than 18 years; in such cases, if the age cut was 14 years, those above the cut-off were considered as adults, but if the cut-off was less the 14, the respective study population was classified as general age. Moreover, if a study population’s age range surpassed 65, the group was classified as adults. This means that in certain situations, the adult population could include elderly patients. However, if any study group contained both elderly and pediatric patients (i.e. less than 14 years), or the age specific epidemiology could not be definitely derived, the report was considered as a general age group. Additionally, in general, the cut-off age for defining elderly patients was 65 years; however, the subclass still included studies where the cut-off point was as low as 60 years. If the age range was less than 60 in its lower boundary, the population was classified as adults.
Trial selections for inclusion into the meta-analyses: Any study with a report of renal syndromes including definitive cases of NiS patients undergoing renal biopsies with a final diagnosis report, discretely or individually, defined for patients with each clinical syndrome (particularly NiS) were considered eligible for inclusion. No quality control criteria more than the abovementioned was used to include or exclude the studies identified.
Data extraction, data set preparations, and accuracy check (twice) were done by the author. The information extracted from each study were as follows: author, publication year, time and duration of the study, country, region/province/town, nephrology center(s), range (or mean ± SD) of age, incidence of NiS in all renal biopsy population, cases of NiS-NS, and final diagnoses of renal biopsies due to NiS. All studies that had been representing their epidemiological data for NiS and associated diagnosis without significant skewed selection in their series reports were considered eligible for entering the meta-analysis without more scrutiny in the study quality assessment.
More detailed methodology of data synthesis and meta-analyses has been published previously. Final renal diagnoses have been extracted as dichotomous data (e.g., MGN yes/no) and analyses have been reported as proportions with 95% confidence intervals (CIs, truncated at 0 and 1) from the extracted data. The study results were then stratified by the reports’ age subgroups (i.e. pediatric, adult, elderly, general), and global regions of the reviewed studies (i.e. East Asia, Europe, Latin America, Middle East, South Asia, United States-Australia; no report from sub-Saharan Africa). A random effect model was employed in order to pool outcome event rates using Stata v.9.0 software (StataCorp LP). Statistical heterogeneity between summary data was assessed using the Cochrane I2 statistic. SPSS software for Windows 15.0 (SPSS Inc.) and Microsoft Excel 2013 were used wherever needed.
Table 1 summarizes characteristics of the reviewed reports. Overall, 26414 patients with NiS have been identified from a total of 96738 patients undergoing a renal biopsy procedure reported by 47 studies from 23 countries, and their data have been reviewed and analyzed. China with 13581 NiS patients (out of a total number of 35523 cases undergone renal biopsy for any reason) contributed the largest share (51.4%) of the pooled NiS cases in this review, followed by Japan and The Czech Republic [4629 (17.5%) and 2728 (10.3%), respectively]. The frequency (95%CI) of NiS as the indication for renal biopsy was 21% (20.7-21.2) for the reviewed studies, ranging from 8% (7.5-8.5) in South Asia to as high as 36.3% (35.9-36.8) in East Asia (Figure 2A). Pediatric patients represented the lowest frequency (95%CI) of NiS as the indication for renal biopsy [7% (5.8-8.2)] while the general age group represented the highest [25.1% (24.6-25.5)] (Figure 2B). The single highest prevalence of NiS as the indication for renal biopsy in an age-region subgroup was for East Asian patients in the general-age group (Supplementary Figure 1).
| Ref. | Country | Region/town | Nephrology centers | Study duration | Publication year | Age, range/mean ± SD | Total, n |
| Ossareh et al[4] | Iran | Tehran | Hasheminejad Kidney Center | 1998-2007 | 2010 | 12–84 | 1407 |
| Saberafsharian et al[5] | Iran | Mashhad | Ghaem and Emam Reza hospitals | 2016-2018 | 2020 | 41.40 ± 16.02 | 860 |
| Pakfetrat et al[6] | Iran | Shiraz | Shiraz University of Medical Sciences | January 2011–December 2017 | 2020 | 1- 60 | 1355 |
| AlFaadhel et al[7] | Kingdom of Saudi Arabia | Riyadh and Jeddah | Hospital, Jeddah; Security Forces Hospital, Riyadh; College of Medicine, King Saud University, Riyadh | 1998-2017 | 2019 | 18-65 | 1070 |
| Al-Saegh et al[8] | Iraq | Kerbala | University Hospital of Kerbala | June 2010-June 2012 | 2013 | 6–50 | 58 |
| Ismail et al[9] | Egypt | Zagazig | Zagazig University | June 2012-November 2014 | 2016 | 16-70 | 150 |
| Al-Qaise et al[10] | Jordan | Amman | Princess Iman Research and Laboratory Center, King Hussein Medical Center | January 2005-December 2008 | 2010 | 14-75 | 273 |
| Turkmen et al[11] | Turkey | Nation-wide data | 47 centers across Turkey | May 2009-May 2019 | 2020 | 41.5 ± 14.9 | 4399 |
| Sahinturk et al[12] | Turkey | Antalya | Antalya Training and Research Hospital | 2006-2016 | 2019 | > 65 yr | 136 |
| Hu et al[13] | China | Henan | The First Affiliated Hospital of Zhengzhou University | January 2009-December 2018 | 2020 | ≤ 14–60+ | 34,630 |
| Su et al[14] | China | Changchun | First Hospital of Jilin University | January 2007-December 2016 | 2019 | > 14 yr | 2725 |
| Wang et al[15] | China | Xinxiang | The First Affiliated Hospital, Xinxiang Medical University | January 1996–December 2010 | 2013 | 16–72 yr | 919 |
| Chiu et al[16] | Taiwan of China | Taichung | Taichung Veterans General Hospital | January 2014-September 2016 | 2018 | 48.4 ± 16.6 | 1445 |
| Nair et al[17] | United States | Nationwide | Multiple referral centers | March 2001-December 2003 | 2004 | 60-91 | 533 |
| Harmankaya et al[18] | Turkey | Istanbul | Bakirkoy Dr. Sadi Konuk Education and Research Hospital | 2006 and 2014 | 2015 | ≥ 65 | 103 |
| Sarwal et al[19] | India (North) | Chandigarh | Post Graduate Institute of Medical Education and Research | 2007 to 2016 | 2019 | 2–94 | 359 |
| Devadass et al[20] | India (South) | Bangalore | M.S. Ramaiah Medical College and Hospitals | 2008 to 2013 | 2014 | 8 mo-78 yr | 680 |
| Das et al[21] | India (South) | Hyderabad | M.S. Ramaiah Medical College and Hospitals | January 1990-December 2008 | 2011 | 10-80 | 1849 |
| Gupta et al[22] | India | New Delhi | Sir Ganga Ram Hospital | January 2011-December 2014 | 2018 | 60–85 | 109 |
| Mohapatra et al[23] | India | Vellore | Christian Medical College and Hospital | January 1996-December 2015 | 2018 | 12.8 ± 4.9 | 1740 |
| Modugumudi et al[24] | India | Tirupati | Sri Venkateswara Institute of Medical Sciences | May 2010-August 2012 | 2016 | 15-74 | 137 |
| Khetan et al[25] | India | Hyderabad | Apollo Hospitals, Jubilee Hills | N/A | 2018 | 0-15 | 799/958 |
| Beniwal et al[26] | India | Jaipur, Rajasthan | SMS Medical College and Hospital | January 2012-December 2017 | 2020 | 60-87 | 230 |
| Koshy et al[27] | India | Chennai, Tamil Nadu | Madras Medical Institute | January 2010-August 2016 | 2018 | 60-82 | 231 |
| Maixnerova et al[28] | Czech | National report | 31 centers | 1994–2011 | 2014 | 0-75+ | 10472 |
| Horvatic et al[29] | Croatia | Zagreb | Dubrava University Hospital | 1996 till February 2012 | 2013 | 16-84 | 922 |
| Oygar et al[30] | Cyprus | North Cyprus | Burhan Nalbantoglu General Hospital | January 2006-2015 | 2017 | 18-78 | 153 |
| Perkowska-Ptasinska et al[31] | Poland | National | The Polish Registry of Renal Biopsies | 2009-2014 | 2017 | 19-88 | 8443-951 = 7492 |
| Pio et al[32] | Portugal | Porto | Hospital Geral de Santo António | January 1997-December 2008 | 2010 | 1 mo-18 yr | 142 |
| Naumovic et al[33] | Serbia | Belgrade | University of Belgrade | 1987 to 2006 | 2009 | 16-79 | 1733 |
| Volovăt et al[34] | Romania | Iasi | “Dr. C. I. Parhon” Hospital | 2005-2010 | 2013 | 41.9 ± 2.8 | 514/559 |
| Covic et al[35] | Romania | Timisoara | C.I. Parhon’ Hospital, Iasi and 2 Dialysis and Transplantation Centers | 1995–2004 | 2006 | 18–80 | 635 |
| Costa et al[36] | Brazil (NorthEast) | Pernambuco | 2 centers: Hospital das Clínicas da Universidade Federal de Pernambuco (HC-UFPE) and Instituto de Medicina Integral Professor Fernando Figueira (IMIP) | February 1998-January 2016 | 2017 | 0-60+ | 677/1151 |
| Özkayin et al[37] | Turkey | Edirne | Trakya University School of Medicine | 2005-2015 | 2016 | 1-17 | 100 |
| Sugiyama et al[38] | Japan | National registry report | 94 centers | January 2009-December 2010 | 2013 | 0-80+ | 7034 |
| Sugiyama et al[39] | Japan | Nationwide | 23 centers | 1979 and 2008 | 2011 | 0–80+ | 2404 |
| Malik et al[40] | Pakistan | Bahawalpur | Bahawal Victoria Hospital | January 2012-April 2018 | 2019 | 14-68 | 195 |
| Imtiaz et al[41] | Pakistan | Karachi | The Kidney Center Post Graduate Training Institute | January 1996-December 2013 | 2017 | 18–88 | 1521 |
| Hashmi et al[42] | Pakistan | Karachi | Liaquat National Hospital | January 2009-December 2013 | 2016 | 20-75 | 140 |
| Mubarak et al[43] | Pakistan | Karachi | Sindh Institute of Urology and Transplantation | July 1995-December 2008 | 2011 | 19–85 | 1793 |
| Imtiaz et al[44] | Pakistan | Karachi | The Kidney Center Post Graduate Training Institute | 1997 to 2013 | 2016 | 0.1-17 | 423 |
| Lanewala et al[45] | Pakistan | Karachi | Sindh Institute of Urology and Transplantation | July 1995 and June 2008 | 2009 | 4 mo-18 yr | 801 |
| AlYousef et al[46] | Kuwait | Sabah Al Nasser | Farwaniya Hospital | January 2013-December 2018 | 2020 | 12-90 | 545 |
| Mesquita et al[47] | Belgium | Brussels | Brugmann University Hospital | January 1991-December 2006 | 2011 | Adult (47 ± 19) | 326 |
| Jegatheesan et al[48] | Australia | Queensland | 11 hospitals | January 2002-December 2011 | 2016 | 48 ± 17 (18+) | 2048/3697 |
| Prada Rico et al[49] | Colombia | Bogot´a, Cundinamarca | Fundaci´on Cardioinfantil, Bogot´ | 2007-2017 | 2013 | 11 ± 4.3 | 241 |
Table 2 summarizes meta-analyses results of the final diagnosis epidemiology in NiS patients regarding the reports’ global continental regions (Supplementary Figures 2-19 represent the forest plots). As is evident from the table and figures, the single most likely renal diagnosis to be made in NiS patients is IgA nephropathy (38.3%), followed by the lupus nephritis (8.2%) and HSP (7.1%).
| Nephropathy | Highest rate (%) | Lowest rate (%) | Pediatric (%) | Adults (%) | Elderly (%) | General (%) | NiS-NS (%) | Total (%) |
| MGN | M.E. 10.2 (8.1-12.3) | Eu. 2.4 (1.9-2.8) | 2.5 (0.4-4.6) | 7.3 (6.9-7.7)1 | 2.3 (0-5.7) | 4.4 (3.9-4.8) | 11.7 (6.8-16.6) | 5.9 (5.6-6.2) |
| IgA nephropathy | E.A. 50.1 (49.3-50.8) | S.A. 9.8 (7.6-11.2) | 11 (8.2-13.7) | 42.6 (41.9-43.4)1 | 5.9 (2.8-8.9) | 37.4 (36.4-38.3) | 3.7 (0-7.8) | 38.3 (37.7-38.9) |
| Henoch Schönlein purpura2 | Eu. 10.7 (2.8-18.6) | S.A. 1.9 (0.5-3.2) | 6.3 (3-9.6) | 7.6 (7.2-8.1)1 | - | 1.2 (0-2.6) | - | 7.1 (6.6-7.5) |
| FSGS | M.E. 11.4 (9.3-13.4) | E.A. 1.6 (1.4-1.8) | 3.4 (1.7-5.1) | 1.6 (1.4-1.8) | 3.9 (0.9-6.8) | 4.3 (3.9-4.7)1 | 19.4 (13-25.8) | 2.1 (1.9-2.2) |
| Lupus nephropathy | L.A. 44.6 (33.7-55.5) | Eu. 4.6 (4-5.3) | 12.9 (9.8-15.9)1 | 9.3 (8.9-9.8) | 5.3 (1.6-8.9) | 5.4 (4.7-6.1) | 10.4 (6.1-14.7) | 8.2 (7.8-8.6) |
| MCD | S.A. 4.4 (1.8-6.9) | E.A. 0.7 (0.5-0.8) | 5.7 (0-12.6)1 | 0.7 (0.6-0.8) | - | 1.6 (1.2-1.9) | - | 0.8 (0.7-0.9) |
| Crescentric GN | USCA 18.9 (16.6-21.3) | E.A. 0.6 (0.2-1) | 3.4 (1.7-5) | 1.7 (1.3-2.2) | 45.7 (36.6-54.8)1 | 6.4 (5-7.9) | - | 2.3 (1.9-2.7) |
| MPGN | USCA. 12.9 (4.8-20.9) | E.A. 0.9 (0.7-1.1) | 14.2 (11.4-17) | 1 (0.9-1.2) | 17.5 (12.1-22.9)1 | 4.1 (3.5-4.8) | 9.2 (4.2-13.5) | 1.3 (1.1-1.4) |
| Amyloidosis | Eu. 1.2 (0.5-1.9) | E.A. 0.8 (0.6-1.1) | 0.6 (0-1.4) | 0.4 (0.1-0.7) | - | 2 (1.6-2.4)1 | - | 0.9 (0.7-1.1) |
| Diabetic nephropathy | Eu. 3.9 (3.3-4.5) | S.A. 0.8 (0-1.6) | - | 1.5 (1.3-1.7) | 3.1 (0-6.2)1 | 2.7 (2.2-3.2) | - | 1.7 (1.5-1.9) |
| TID | L.A. 27.8 (4.9-50.7) | E.A. 0.6 (0.5-0.7) | 3.5 (1.1-5.8) | 0.6 (0.5-0.8) | 6.7 (1.8-11.7)1 | 2.3 (1.3-3.3) | - | 0.7 (0.5-0.8) |
| Vascular nephropathy | L.A. 19.3 (10.6-27.9) | M.E. 0.8 (0.1-1.5) | 2.9 (0.4-5.4) | 2.2 (1.9-2.4) | 4.3 (1.4-7.2)1 | 3 (2.5-3.5) | - | 2.3 (2.1-2.5) |
| Nephroangiosclerosis2 | M.E. 20 (0-57.8) | S.A. 0.7 (0-1.6) | - | 1.7 (1.5-1.9) | 22.7 (9.8-35.6)1 | 3.3 (2.7-3.9) | - | 1.8 (1.6-2) |
| Hereditary nephropathy | Eu. 3.4 (0.9-5.9) | E.A. 0.7 (0.6-0.9) | 2.9 (0.8-5)1 | 0.7 (0.6-0.9) | - | - | - | 0.8 (0.6-0.9) |
| Unspecific Proliferative GN | S.A. 34.2 (31.5-37) | E.A. 1.4 (1.2-1.6) | 23.4 (20-26.9)1 | 1.6 (1.4-1.8) | 20.4 (9.7-31) | 11.7 (9.8-13.6) | 14.1 (9-19.2) | 1.7 (1.6-1.9) |
| MesPGN2 | E.A. 10 (8.2-11.8) | S.A. 4.5 (3.1-5.9) | 7.5 (5.2-9.7)1 | 5.3 (4.5-6.2) | - | 6.2 (4.5-8) | 9.2 (4.2-13.5) | 5.7 (5-6.5) |
| Unspecific Paraproteinemia | S.A. 11.8 (1.6-22) | E.A. 0.6 (0.4-0.7) | - | 0.6 (0.4-0.7) | 11.8 (1.6-22)1 | - | - | 0.6 (0.4-0.7) |
There were profound disparities in the epidemiology of diagnoses regarding the reports’ global regions. For example, the possibility of diagnosing unspecific PPEs in NiS patients from South Asia is about 20 times more than that for the East Asia (Table 2). MGN and FSGS were more frequently diagnosed in NiS patients from the Middle East, while in the South Asia, unspecific PPEs, as well as PGN, were by far the most likely diagnoses compared to the other world regions. In East Asia, as expected, IgA nephropathy, and MesPGN were the most likely diagnoses, together comprising over 60% of all the diagnoses made for NiS patients; whereas both entities were the least likely ones to be reported in the South Asia. Hereditary nephropathy, diabetic nephropathy, amyloidosis, and HSP were relatively more frequent in the European NiS patients, while in the USCA region, MPGN and CresGN were the relatively predominant diagnoses (Table 2).
As mentioned for the world regions, there has also been disparity in the epidemiology of renal disease diagnoses in NiS patients regarding their age subgroups (Table 2 summarizes results of the respective meta-analyses, and Supplementary Figures 20-35 illustrate the forest plots). Relative to the pediatric and elderly patient groups presenting with NiS, adults were significantly more likely to be diagnosed with IgA nephropathy, HSP, and MGN. Among these, the disparity was most prominent for IgA nephropathy (only 11% and 6% of pediatric and elderly patients with NiS, respectively, were finally diagnosed with IgA nephropathy vs about 43% for the adults). On the other hand, pediatric NiS patients were more frequently diagnosed with lupus nephritis, MCD, hereditary nephropathy, MesPGN, and unspecific PGN, with the relatively largest disparity found with MCD. Finally, elderly patients were more likely to get diagnoses with CresGN, MPGN, TID, unspecific PPEs, diabetic nephropathy, and vascular nephropathy (including NAS), among which CresGN, unspecific PPEs and NAS were by far more frequent in this age group (vs the younger ones).
Another interesting observation in the study of the age-groups was that there was a trend towards higher or lower frequencies in the rates of diagnoses based on the subgroups’ ages. For example, while lupus nephritis, MCD and MesPGN were decreasing in the frequency of diagnosis by advances in age (pediatrics > adults > elderly), CresGN, diabetic nephropathy, vascular nephropathy (and NAS), and unspecific PPEs were increasing by age. This observation might more strongly recommend the age effect on the occurrence of the respective renal diseases.
To further subclassify the patients according to their epidemiological characteristics in order to find the ones at the highest risks for each renal entity, the reports have been categorized simultaneously upon their age and world region. Supplementary Figures 36-42 summarize the results. As is depicted in Supplementary Figure 36, among all the other age and region subgroups, MGN was most frequently diagnosed in adults with NiS from the East Asia, comprising 13% of all the diagnoses. Likewise, IgA nephropathy was also most prevalently diagnosed among the East Asian adults, which together with MGN, comprise about 60% of all the diagnoses in this subgroup of NiS patients (Supplemen
Elderly Americans (54%) and elderly Europeans (34%) presenting with NiS were most likely to be finally diagnosed with the crescentric nephropathy, followed by the adult Australians and adult Europeans (17% each, Supplementary Figure 40). MPGN was the predominant diagnosis among the South Asian elderly (27%), followed by the European pediatrics (23%) and South Asian pediatrics and adults (14% each). This suggests that patients in South Asia presenting with NiS are at a substantial risk of MPGN diagnosis, irrespective of their age. But MPGN was not the only renal diagnosis frequently found in the South Asia (Supplementary Figure 41). Unspecific PGN was most frequently found in the general age South Asians (47%, Supplementary Figure 42), which together with MPGN, it suggests South Asia as a main source of diagnosing PGN among NiS patients.
Three of the reviewed studies had discriminately reported their series with patients representing NiS-NS, and the epidemiology of their final diagnosis has been compared to that of the NiS-alone patients. As summarized in Table 2 and illustrated in the Supplementary Figures 43-50, NiS-NS patients represented higher diagnosis rates for MGN, FSGS, MPGN, MesPGN, and unspecific PGN than NiS-alone patients, while representing a lower frequency of IgA nephropathy. Lupus nephritis was comparably observed between the two groups.
This study in all probability represents the literature with the single most comprehensive overview of NiS as the indication for renal biopsy procedure, the expected diagnoses, and predictive factors. The overall frequency of NiS as the indication for renal biopsies was about 21% of the total reports, with the highest rates in East Asia comprising over one third of all the cases (Japan represented the single highest frequency) and lowest in the South Asia (8%, Figure 2A). Patients of the general and adult age groups were the most likely age subgroups receiving kidney biopsies due to NiS, while pediatrics represented the lowest frequency of NiS as the indication for renal biopsies (Figure 2B).
Compared to the other renal syndromes, this study showed that NiS is associated with significant bias in the frequency of different final diagnoses. Some of the renal diagnoses (including proliferative endocapillary glomerulonephritis, hepatitis B virus nephropathy, IgM nephropathy, and minor glomerular abnormalities) were in such scarcity in NiS patients that this led to their exclusion from the final report, while some of which were quite frequent diagnoses in patients with other renal syndromes[2,3,50]. MGN was a dominant diagnosis in nephrotic syndrome patients comprising about 20% of the total population[2], however, this rates in the sub-nephrotic proteinuria[3] and NiS (current report), were much lower (7.5% and 6%, respectively). On the other hand, IgA nephropathy was the most likely diagnosis in NiS patients comprising over one third of all the diagnoses, while these rates for the sub-nephrotic proteinuria and nephrotic syndrome were much less (17% and 4.5%, respectively)[2,3].
The global disparities in the epidemiology of the final diagnoses being made on renal biopsies of patients representing with any renal syndrome is also of extreme interest. For example, a previous systematic review has demonstrated that IgA nephropathy is most prevalent in the East Asia, comprising more than one third of all the diagnoses made for patients undergoing renal biopsies for any indication. However, this will be of limited practical relevance due to the profound disparity in diagnoses expected for different renal syndromes. For instance, the incidence of IgA nephropathy in the East Asia as reported by the current study was roughly 50% for NiS patients, far more than its overall frequency reported for the same region when estimated irrespective of the clinical syndrome (approximately 35%); similar observations have been made for nephrotic syndrome and sub-nephrotic proteinuria in the previous systematic reviews[2,3].
The next region representing a highly skewed frequency for a specific diagnosis was Latin America for lupus nephritis (approximately 44%); interestingly, considering the same concept for nephrotic syndrome and sub-nephrotic proteinuria, Sub-Saharan Africa and the Middle East were, respectively, the predominant regions of high frequency (approximately 12% and approximately 14%), with the former having no representative patients in the current review study on NiS.
A profound discrepancy has also been detected in the frequency of renal diagnoses regarding the reports’ age groups. While MCD, lupus nephritis, hereditary nephropathy, MesPGN, and unspecific PGN made the predominant diagnoses in the pediatric NiS patients, about 43% of adults were finally diagnosed with IgA nephropathy. A similar observation was observed for the elderly population with over 45% of them being diagnosed with crescentric nephropathy. Predictably, the elderly population was the predominant age subgroup for the diagnosis of vascular nephropathies (including NAS), TID, diabetic nephropathy, and PPEs. Here again, a profound bias has been detected in the epidemiology of renal diagnoses regarding the clinical syndromes. For example, for nephrotic syndrome[2], about half of the pediatric patients were ultimately diagnosed with MCD, while this percentage was about 8% for sub-nephrotic proteinuria[3], and 6% for NiS patients (current study). Detection of MCD such a high percentage of pediatric patients with NiS is a considerable finding and changes presumptions. The next substantial disparity was detected for MGN in the elderly, with 35%, approximately 19% and 2.3% rates of diagnosis, respectively, for nephrotic, sub-nephrotic, and NiS (2, 3 and current study).
Meta-analyses from the current study have also revealed age-dependent disparities in the frequencies of final diagnoses. For example, the frequency of IgA nephropathy in NiS patients was by far highest among adults, while in the contexts of nephrotic syndrome or sub-nephritic proteinuria, pediatric patients were the age subclass most likely to be diagnosed with the entity, with a decreasing trend being detected with increases in the age subclasses (lower for adults and then the lowest in the elderly)[2,3].
Subcategorization of the reports simultaneously for their age and the global regions also revealed some very interesting and unprecedented observations. Two of the most interesting findings were the high rates of diagnosing crescentric nephropathy in various age subclasses from regions with the majority white ethnicity (Europe, United States, and Australia), as well as South Asia being the leading source of MPGN diagnosis in all their age subgroups; both the abovementioned suggest high levels of ethnic liability, environmental predispositions, and life-style effects on the epidemiology of renal diseases even within the same clinical syndromes.
Another subject of analysis in this study was the NiS-NS subgroup whose clinical syndrome included NiS with nephrotic range proteinuria that had been reported in a subgroup of patient populations by some of the reviewed studies. A comparison of NiS-NS epidemiological findings with the respective results from subnephrotic proteinuria, NiS-(alone) and nephrotic syndromes suggests that NiS-NS patients exhibit considerable disparities in the frequencies of renal diagnoses, proposing NiS-NS as a new syndrome entity. Although the limited sample size, as well as the disparities in other potential intervening factors, could confound the conclusion.
The findings of the current study are associated with limitations. The limited number of reports from specific regions of the world, the small sample sizes for each study and occasionally selection deviations in some of the studies (e.g., age specific reports) were the most important limitations. For example, a finding of this study was the preponderance of crescentric nephropathy as the final diagnosis of NiS patients for both the elderly patients among the age subgroups and United States-Australia regarding the regional analyses. Together, it is conceivable that the observed high frequency of crescentric nephropathy diagnosis reported for the latter might in part be due to the potential inclusion of relatively older patients compared to the reports from the other global regions. Finally, sub-Saharan Africa had no representative in this review, and therefore the results of this study might not be well applied to patients from this region/ethnicity.
In conclusion, NiS, as the indication for renal biopsy, represents a very distinctive epidemiology of renal diagnoses than those of other major syndromes. Within the NiS group, there is a wide spectrum of epidemiological variations regarding the age subclasses as well as the regions of studies. Understanding of these disparities helps the researchers, clinicians, and the health care systems in the management of their patients, and helps societies plan the best way to assign available resources to the areas that might promise more health advantages. It also provides motivations for future research to find the reasons behind the reported disparities and to intervene accordingly.
Nephritic syndrome (NiS) is a major indicator of severe kidney disease requiring renal biopsy for histopathological evaluation, but limited understanding of the syndrome and its significance is currently lacking due to the lack of a comprehensive review in the literature.
The current understanding on the epidemiology of renal diseases finally diagnosed in patients representing various clinical syndromes as indications for the renal biopsy is inaccurate and skewed.
This systematic review aims at collecting the available data in the literature to give the most possible comprehensive overview on the epidemiology of diagnoses that we may expect from the evaluations of renal biopsies in patients with nephritic syndrome.
A systematic review of the literature has been conducted, with 47 studies identified for meta-analyses.
A myriad of results have been made through this systematic review, the most important of them is the high prevalence of immunoglobulin A nephropathy (about 38%) as the final diagnosis of nephritic syndrome, and diagnosing minimal change disease in a proportion of pediatric patients representing with NiS.
The diagnostic spectrum of nephritic syndrome is quite wide, and clinicians should have a better overview on all the possibilities.
It has clinical, research and health care perspectives to the society.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bacharaki D, Greece; Yu F, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Heron M. Deaths: Leading Causes for 2017. Natl Vital Stat Rep. 2019;68:1-77. [PubMed] [Cited in This Article: ] |
| 2. | Taheri S. Nephrotic Syndrome & Diagnoses: 2021 Update. N Lahij Med J. 2021;5:13-21. [Cited in This Article: ] |
| 3. | Taheri S. Sub-Nephrotic Proteinuria as the Indication for a Kidney Biopsy: Review. Cytol Histol Int J. 2021;5:000134. [DOI] [Cited in This Article: ] |
| 4. | Ossareh S, Asgari M, Abdi E, Nejad-Gashti H, Ataipour Y, Aris S, Proushani F, Ghorbani G, Hayati F, Ghods AJ. Renal biopsy findings in Iran: case series report from a referral kidney center. Int Urol Nephrol. 2010;42:1031-1040. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Saberafsharian M, Ravanshad S, Hami M, Ghorban Sabbagh M, Sanei E, Miri M. The Spectrum of Glomerular Diseases in Mashhad According to Kidney Biopsy Records. Iran J Kidney Dis. 2020;14:184-190. [PubMed] [Cited in This Article: ] |
| 6. | Pakfetrat M, Malekmakan L, Torabinezhad S, Yousefi O, Naddaffard D. Review of Renal Biopsies, A Single Center Experience. Iran J Kidney Dis. 2020;14:12-19. [PubMed] [Cited in This Article: ] |
| 7. | AlFaadhel T, Alsuwaida A, Alsaad K, Almezaini L, Ahmed N, AlHamad MY, Bakheet A, Wadera J, Mokhtar G, Alsuwaida F, Siddiqui R, Kechrid M, Abdelrehman A, Husain S, Kfoury H, Alabdulsalam A, Alanazi M, Oudah NA, AlHozali H. Prevalence and 20-year epidemiological trends of glomerular diseases in the adult Saudi population: a multicenter study. Ann Saudi Med. 2019;39:155-161. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Al-Saegh RM, Assad LW. The spectrum of glomerular diseases as studied by immunofluorescence microscopy a single center study in Iraq. Arab J Nephrol Transplant. 2013;6:161-167. [PubMed] [Cited in This Article: ] |
| 9. | Ismail MI, Lakouz K, Abdelbary E. Clinicopathological correlations of renal pathology: A single center experience. Saudi J Kidney Dis Transpl. 2016;27:557-562. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Al-Qaise N, Qdah A, Haddad A. Spectrum of Glomerular Diseases at King Hussein Medical Center. J Royal Med Serv. 2021;. [DOI] [Cited in This Article: ] |
| 11. | Turkmen A, Sumnu A, Cebeci E, Yazici H, Eren N, Seyahi N, Dilek K, Dede F, Derici U, Unsal A, Sahin G, Sipahioglu M, Gok M, Tatar E, Dursun B, Sipahi S, Yilmaz M, Suleymanlar G, Ulu S, Gungor O, Kutlay S, Bahcebasi ZB, Sahin I, Kurultak I, Turkmen K, Yilmaz Z, Kazancioglu RT, Cavdar C, Candan F, Aydin Z, Oygar DD, Gul CB, Arici M, Paydas S, Taymez DG, Kucuk M, Trablus S, Turgutalp K, Koc L, Sezer S, Duranay M, Bardak S, Altintepe L, Arikan IH, Azak A, Odabas AR, Sahin GM, Ozturk S. Epidemiological features of primary glomerular disease in Turkey: a multicenter study by the Turkish Society of Nephrology Glomerular Diseases Working Group. BMC Nephrol. 2020;21:481. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Sahinturk Y, Sarikaya M, Dolu S, Kok M, Inci A, Caliskan AR. The aging kidney: A 10-year renal biopsy study of geriatric population. Ann Med Res. 2019;1. [DOI] [Cited in This Article: ] |
| 13. | Hu R, Quan S, Wang Y, Zhou Y, Zhang Y, Liu L, Zhou XJ, Xing G. Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep. 2020;10:10994. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Su S, Yu J, Wang Y, Li J, Xu Z. Clinicopathologic correlations of renal biopsy findings from northeast China: A 10-year retrospective study. Medicine (Baltimore). 2019;98:e15880. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Wang YT, Zhou CY, Zhu TC, Yang J, Zhang Y, Xu QY, Guo MH. Analysis of kidney biopsy data from a single center in the midland rural area of china, 1996-2010. Curr Ther Res Clin Exp. 2013;74:22-25. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Chiu HF, Chen HC, Lu KC, Shu KH; Taiwan Society of Nephrology. Distribution of glomerular diseases in Taiwan: preliminary report of National Renal Biopsy Registry-publication on behalf of Taiwan Society of Nephrology. BMC Nephrol. 2018;19:6. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Nair R, Bell JM, Walker PD. Renal biopsy in patients aged 80 years and older. Am J Kidney Dis. 2004;44:618-626. [PubMed] [Cited in This Article: ] |
| 18. | Harmankaya O, Okuturlar Y, Kocoglu H, Kaptanogullari H, Yucel SK, Ozkan H, Acarer D, Erdogan E, Yilmaz M, Hursitoglu M. Renal biopsy in the elderly: a single-center experience. Int Urol Nephrol. 2015;47:1397-1401. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Sarwal D, D'Cruz S, Singh Punia RP, Minz RW. The spectrum of renal diseases observed in native renal biopsies in a single North Indian tertiary care center. Saudi J Kidney Dis Transpl. 2019;30:492-500. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Devadass CW, Mysorekar VV, Gireesh MS, Mahesh E, Gurudev KC, Radhika K. Review of renal biopsy database: A single centre South Indian study. Inter J Med Res Heal Sci. 2014;3:4. [DOI] [Cited in This Article: ] |
| 21. | Das U, Dakshinamurty KV, Prayaga A. Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol. 2011;21:250-257. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Gupta P, Rana DS. Importance of renal biopsy in patients aged 60 years and older: Experience from a tertiary care hospital. Saudi J Kidney Dis Transpl. 2018;29:140-144. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Mohapatra A, Kakde S, Annapandian VM, Valson AT, Duhli N, Korula A, Matthai SM, Pulimood AB, David VG, Alexander S, Jacob S, Varughese S, Basu G, Tamilarasi V, John GT. Spectrum of biopsy proven renal disease in South Asian children: Two decades at a tropical tertiary care centre. Nephrology (Carlton). 2018;23:1013-1022. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Modugumudi AS, Venkata PB, Bottla SK, Kottu R, Nandyala R, Patnayak R, Chowhan AK, Yadgiri LA. A study of primary glomerular diseases in adults; clinical, histopathological and immunofluorescence correlations. J Nephropharmacol. 2016;5:91-97. [PubMed] [Cited in This Article: ] |
| 25. | Khetan K, Gupta G, Swarnalata G. Study of paediatric renal biopsies with clinicopathologic correlation and comparison with literature on adult renal biopsies. Indian J Cancer. 2018;5:97-105. [DOI] [Cited in This Article: ] |
| 26. | Beniwal P, Singh SK, Malhotra V, Agarwal D, Sharma M, Joshi P, Khandelwal S, Gaur N, Sharma S. Gerontolizing Nephrology: Spectrum of Histopathological Findings of Kidney Biopsy in the Elderly. Indian J Nephrol. 2020;30:264-269. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Koshy PJ, Parthsarathy R, Mathew M, Prabakaran R, Kuruvilla S, Abraham G. Interpretation of Kidney Biopsy in Indian Patients Older than 60 Years: A Tertiary Care Experience. Indian J Nephrol. 2018;28:198-202. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Maixnerova D, Jancova E, Skibova J, Rysava R, Rychlik I, Viklicky O, Merta M, Kolsky A, Reiterova J, Neprasova M, Kidorova J, Honsova E, Tesar V. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994-2011. J Nephrol. 2015;28:39-49. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Horvatic I, Tisljar M, Bulimbasic S, Bozic B, Galesic Ljubanovic D, Galesic K. Epidemiologic data of adult native biopsy-proven renal diseases in Croatia. Int Urol Nephrol. 2013;45:1577-1587. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Oygar DD, Neild GH. Reporting renal biopsies from Cyprus: a systematic approach. J Nephropathol. 2017;6:231-239. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Perkowska-Ptasinska A, Bartczak A, Wagrowska-Danilewicz M, Halon A, Okon K, Wozniak A, Danilewicz M, Karkoszka H, Marszalek A, Kowalewska J, Mroz A, Korolczuk A, Oko A, Debska-Slizien A, Naumnik B, Hruby Z, Klinger M, Ciechanowski K, Myslak M, Sulowicz W, Rydzewski A, Wiecek A, Manitius J, Gregorczyk T, Niemczyk S, Nowicki M, Gellert R, Stompor T, Wieliczko M, Marczewski K, Paczek L, Rostkowska O, Deborska-Materkowska D, Bogdanowicz G, Milkowski A, Durlik M; Polish Society of Nephrology. Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol Dial Transplant. 2017;32:ii209-ii218. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Pio D, Figueiredo S, Silva P, Nunes S, Costa T, Carvalho E, Vizcaíno JR, Faria MS, Mota C. Renal biopsies in children. RMMG. 2019;29. [DOI] [Cited in This Article: ] |
| 33. | Naumovic R, Pavlovic S, Stojkovic D, Basta-Jovanovic G, Nesic V. Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant. 2009;24:877-885. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Volovăt C, Cãruntu I, Costin C, Stefan A, Popa R, Volovăt S, Siriopol D, Voroneanu L, Nistor I, Segall L, Covic A. Changes in the histological spectrum of glomerular diseases in the past 16 years in the North-Eastern region of Romania. BMC Nephrol. 2013;14:148. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Covic A, Schiller A, Volovat C, Gluhovschi G, Gusbeth-Tatomir P, Petrica L, Caruntu ID, Bozdog G, Velciov S, Trandafirescu V, Bob F, Gluhovschi C. Epidemiology of renal disease in Romania: a 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant. 2006;21:419-424. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Costa DM, Valente LM, Gouveia PA, Sarinho FW, Fernandes GV, Cavalcante MA, Oliveira CB, Vasconcelos CA, Sarinho ES. Comparative analysis of primary and secondary glomerulopathies in the northeast of Brazil: data from the Pernambuco Registry of Glomerulopathies - REPEG. J Bras Nefrol. 2017;39:29-35. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Özkayın N, Çıplak G, Usta U, Gençhellaç H, Temizöz O. Assessment of Ten-Year-Long Results of Kidney Biopsies Performed on Children in the Thrace Region of Turkey. Balkan Med J. 2016;33:589-593. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, Tsuruya K, Kiyomoto H, Iida H, Sasaki T, Higuchi M, Hattori M, Oka K, Kagami S, Kawamura T, Takeda T, Hataya H, Fukasawa Y, Fukatsu A, Morozumi K, Yoshikawa N, Shimizu A, Kitamura H, Yuzawa Y, Matsuo S, Kiyohara Y, Joh K, Nagata M, Taguchi T, Makino H; Committee for Standardization of Renal Pathological Diagnosis; Committee for Kidney Disease Registry; Japanese Society of Nephrology. Japan Renal Biopsy Registry and Japan Kidney Disease Registry: Committee Report for 2009 and 2010. Clin Exp Nephrol. 2013;17:155-73. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, Tsuruya K, Kiyomoto H, Iida H, Sasaki T, Higuchi M, Hattori M, Oka K, Kagami S, Nagata M, Kawamura T, Honda M, Fukasawa Y, Fukatsu A, Morozumi K, Yoshikawa N, Yuzawa Y, Matsuo S, Kiyohara Y, Joh K, Taguchi T, Makino H; Committee for Standardization of Renal Pathological Diagnosis and Working Group for Renal Biopsy Database, Japanese Society of Nephrology, Tokyo, Japan. Japan Renal Biopsy Registry: the first nationwide, web-based, and prospective registry system of renal biopsies in Japan. Clin Exp Nephrol. 2011;15:493-503. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Malik SI, Idrees MK, Naseem K, Sadiq S, Raza SH, Ahmad FU. Pattern of biopsy-proven kidney diseases: experience of a teaching hospital in Bahawalpur, Pakistan. Saudi J Kidney Dis Transpl. 2019;30:1144-1150. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Imtiaz S, Drohlia MF, Nasir K, Salman B, Ahmad A. Analysis of renal diseases detected in renal biopsies of adult patients: A single-center experience. Saudi J Kidney Dis Transpl. 2017;28:368-378. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Hashmi AA, Hussain Z, Edhi MM, Mumtaz S, Faridi N, Khan M. Insight to changing morphologic patterns of glomerulopathy in adult Pakistani patients: an institutional perspective. BMC Res Notes. 2016;9:73. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Mubarak M, Kazi JI, Naqvi R, Ahmed E, Akhter F, Naqvi SA, Rizvi SA. Pattern of renal diseases observed in native renal biopsies in adults in a single centre in Pakistan. Nephrology (Carlton). 2011;16:87-92. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Imtiaz S, Nasir K, Drohlia MF, Salman B, Ahmad A. Frequency of kidney diseases and clinical indications of pediatric renal biopsy: A single center experience. Indian J Nephrol. 2016;26:199-205. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Lanewala A, Mubarak M, Akhter F, Aziz S, Bhatti S, Kazi JI. Pattern of pediatric renal disease observed in native renal biopsies in Pakistan. J Nephrol. 2009;22:739-746. [PubMed] [Cited in This Article: ] |
| 46. | AlYousef A, AlSahow A, AlHelal B, Alqallaf A, Abdallah E, Abdellatif M, Nawar H, Elmahalawy R. Glomerulonephritis Histopathological Pattern Change. BMC Nephrol. 2020;21:186. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Mesquita M, Fosso C, Bakoto Sol E, Libertalis M, Corazza F, Vanden Houte K, Dratwa M. Renal biopsy findings in Belgium: a retrospective single center analysis. Acta Clin Belg. 2011;66:104-109. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Jegatheesan D, Nath K, Reyaldeen R, Sivasuthan G, John GT, Francis L, Rajmokan M, Ranganathan D. Epidemiology of biopsy-proven glomerulonephritis in Queensland adults. Nephrology (Carlton). 2016;21:28-34. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Prada Rico M, Rodríguez Cuellar CI, Fernandez Hernandez M, González Chaparro LS, Prado Agredo OL, Gastelbondo Amaya R. Characterization and Etiopathogenic Approach of Pediatric Renal Biopsy Patients in a Colombian Medical Center from 2007-2017. Int J Nephrol. 2018;2018:9603453. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Taheri S. Nephropathy Statistics: World Report, 2021. N Lahij Med J. 2021;5:1-7. [DOI] [Cited in This Article: ] |