Copyright
©The Author(s) 2015.
World J Virology. May 12, 2015; 4(2): 56-77
Published online May 12, 2015. doi: 10.5501/wjv.v4.i2.56
Published online May 12, 2015. doi: 10.5501/wjv.v4.i2.56
Figure 1 The human immunodeficiency virus type 1, upon entering peripheral circulation, will infect lymphocytes and macrophages.
The viral proteins gp120 and gp41 of HIV-1 bind to the CD4+ receptor and coreceptors, C-C chemokine receptor type 5 and C-X-C chemokine receptor type 4, on the surface of these cells. The lymphocytes T-CD4 that are infected with HIV-1 produce viral particles and may remain in a latent form within circulation. Infected monocytes can directly present antigen to lymphocytes T-CD4, or transform into tissue macrophages. This process stimulates the host inflammatory response and amplifies the production of proinflammatory cytokines and promotes increased cellular oxidative stress. The production of proinflammatory cytokines by macrophages and lymphocytes promotes a decrease in plasma high-density lipoprotein cholesterol by impairing the cholesterol dependent efflux transporter ATP-binding cassette protein A1 in human macrophages. Additionally, viral proteins and proinflammatory cytokines including interleukin-1, interleukin-6, tumor necrosis factor α and interferon gamma stimulate endothelial lipase enzyme and different acute phase proteins, such as serum amyloid A. Viral proteins also exert effects on adipocytes resulting in mitochondrial dysfunction, production of reactive oxygen species, increased insulin resistance, decreased adiponectin, and change the clearance of triglyceride-rich lipoproteins and insulin resistance. Finally, all of the different cellular mechanisms involved and affected by HIV-1 infection promote an increased risk of cardiovascular disease. Source: de Almeida et al[211]. Gp120: Glycoprotein 120; gp41: Glycoprotein 41; CCR5: C-C chemokine receptor type 5; CXCR4: C-X-C chemokine receptor type 4; LT-CD4: Lymphocytes T-CD4; HDL: High-density lipoprotein; ABCA1: ATP-binding cassette protein A1; IL-1: Interleukin-1; IL-6: Interleukin-6; TNFα: Tumor necrosis factor α; IFN-γ: Interferon gamma; TG-RLP: Triglyceride-rich lipoproteins; ROS: Reactive oxygen species; HIV-1: Human immunodeficiency virus type 1.
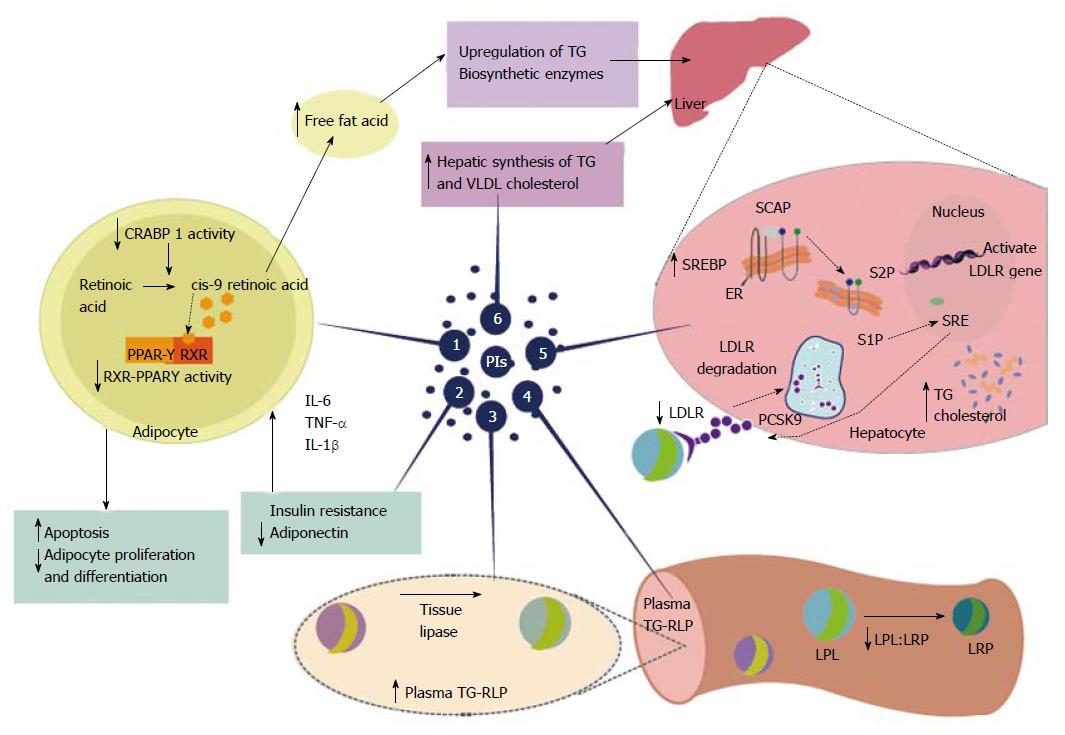
Figure 2 Highly active antiretroviral therapy-associated dyslipidemia is especially evident with the use of protease inhibitors.
Protease inhibitors (PIs) promote a decrease in plasma high-density lipoprotein cholesterol and increased overall cholesterol, triglycerides(TG), and low-density lipoprotein cholesterol. These changes, induced by PIs, promote an increased risk of cardiovascular disease. Proposed mechanisms for PI-based dyslipidemia include the following: (1) There is structural similarity with the amino acid sequence of the C-terminal region of cytoplasmic retinoic acid-binding protein type 1 (CRABP1); thus, the PIs likely bind to CRABP-1, increasing apoptosis and diminishing the proliferation of peripheral adipocytes; (2) PI-mediated increases in the expression and secretion of proinflammatory cytokines, such as tumor necrosis factor alpha, interleukin 1 β and interleukin-6 are involved in altered adipocyte functions and decreased adiponectin; (3-4) PI-induced dyslipidemia is based on the structural similarity between the catalytic region of HIV-1 protease and the LDL-receptor-related protein that interferes with lipoprotein lipase complex formation (LRP-LPL). As a result the adipose storage capacity is reduced and plasma TG-rich lipoproteins are increased; (5) PI suppresses proteasome-mediated degradation of the sterol regulatory element binding proteins (SREBP) in the liver and adipocytes, which are transcription factors responsible for fatty acid and triglyceride synthesis in the liver and adipose tissue and control several steps of cholesterol synthesis. The suppression promotes nSREBP accumulation in the liver and an increase in the biosynthesis of total cholesterol and triglycerides, and adipose tissue, promoting increased insulin resistance, reduced expression of leptin and lipodystrophy; (6) PI-based therapy increases the hepatic synthesis of triglycerides, and to a lesser extent, very-low density lipoprotein cholesterol. Source: de Almeida et al[211]. PIs: Protease inhibitors; HDL: High-density lipoprotein; TG: Triglycerides; LDL: Low-density lipoprotein; CRABP1: C-terminal region of cytoplasmic retinoic acid-binding protein type 1; TNF-α: Tumor necrosis factor alpha; IL-1β: Interleukin 1β; IL-6: Interleukin-6; LRP: LDL-receptor-related protein; LPL: Lipoprotein lipase; TG-RLP: Triglyceride -rich lipoproteins are increased; SREBP: Sterol regulatory element binding proteins; VLDL: Very-low density lipoprotein; RXR-PPARγ: Retinoid X receptor-peroxisome proliferator-activated receptor γ; LDL-R: Low-density lipoprotein-receptor; PCSK9: Proprotein convertase subtilisin-kexin type 9; SCAP; Sterol regulatory element binding protein cleavage activating protein; S1P: Site 1 protease; S2P: Site 2 protease.
- Citation: Cunha JD, Maselli LMF, Stern ACB, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: Old and new drugs. World J Virology 2015; 4(2): 56-77
- URL: https://www.wjgnet.com/2220-3249/full/v4/i2/56.htm
- DOI: https://dx.doi.org/10.5501/wjv.v4.i2.56