Published online Sep 10, 2018. doi: 10.5500/wjt.v8.i5.156
Peer-review started: April 24, 2018
First decision: June 6, 2018
Revised: June 14, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: September 10, 2018
To investigate the specific effects of immunosuppressants on the antiviral action of daclatasvir and asunaprevir.
The antiviral activity of daclatasvir (DCV) and asunaprevir (ASV) combined with immunosuppressants was tested using two in vitro models for hepatitis C virus (HCV) infection.
Tacrolimus, rapamycin and cyclosporine did not negatively affect the antiviral action of DCV or ASV. Mycophenolic acid (MPA) showed additive antiviral effects combined with these direct acting antivirals (DAAs). MPA induces interferon-stimulated genes (ISGs) and is a potent GTP synthesis inhibitor. DCV or ASV did not induce ISGs expression nor affected ISG induction by MPA. Rather, the combined antiviral effect of MPA with DCV and ASV was partly mediated via inhibition of GTP synthesis.
Immunosuppressants do not negatively affect the antiviral activity of DAAs. MPA has additive effect on the antiviral action of DCV and ASV. This combined benefit needs to be confirmed in prospective clinical trials.
Core tip: Since 2013, several new generation direct acting antivirals (DAAs) have been approved for the treatment of hepatitis C virus (HCV), including daclatasvir (DCV) and asunaprevir (ASV). Although a few reports investigated the effectivity of DAAs after liver transplantation, the effects of specific immunosuppressants on the antiviral efficacy remain largely unknown. We investigated the effect of the immunosuppressants on the antiviral action of DCV and ASV in two in vitro models for HCV. We observed that none of the immunosuppressants negatively affected the antiviral activity of these DAAs, and that mycophenolic acid has an additive effect on their antiviral action.
- Citation: de Ruiter PE, Gadjradj Y, de Knegt RJ, Metselaar HJ, Ijzermans JN, van der Laan LJ. Interaction of immunosuppressants with HCV antivirals daclatasvir and asunaprevir: combined effects with mycophenolic acid. World J Transplantation 2018; 8(5): 156-166
- URL: https://www.wjgnet.com/2220-3230/full/v8/i5/156.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i5.156
Liver disease caused by chronic hepatitis C virus (HCV) infection is still the major indication for liver transplantation worldwide. Factors that contribute to the recurrence of HCV after transplantation include viral factors (e.g., HCV RNA levels at the time of transplantation and HCV genotype), host factors (immune response and HCV cryoglobulinemia), and the use of immunosuppressive medication[1].
Glucocorticosteroids like prednisolone are commonly used as immunosuppressant, both as an induction agent to prevent acute rejection and as maintenance immunosuppressive therapy. Some clinical observations suggest that steroid boluses used to treat acute rejection are associated with an increase in HCV viral load and with severity of HCV recurrence. However, no direct effect of prednisolone on HCV replication could be demonstrated in vitro. We have previously shown that prednisolone does not affect the action of direct-acting antivirals against hepatitis C, but that it acts on the antiviral function of plasmacytoid dendritic cells by inhibiting the production of interferon-alpha[2,3].
Calcineurin inhibitors (CNIs) are the most widely prescribed immunosuppressants after liver transplantation. Cyclosporine A (CSA) and tacrolimus (TAC) form complexes with immunophilins, resulting in the inhibition of the activity of calcineurin[4]. CSA can inhibit HCV replication in vitro by blocking the activity of cyclophilins that interact with viral protein NS5B[5,6]. The antiviral action of CSA is independent of calcineurin signaling[7]. CSA also has a broad antiviral activity against Influenza A and B viruses[8]. TAC has no effect on HCV replication[9,10].
Mycophenolic acid (MPA), the active form of mycophenolate mofetil (MMF) is a non-competitive inhibitor of inosine monophosphate dehydrogenase (IMPDH). This protein, in particular the isoform IMPDH2, is crucial for the de novo synthesis of guanosine nucleotides. Next to its immunosuppressive properties, MPA has potent and broad anti-viral activity: replication of rotavirus, influenza, and hepatitis E virus[11-13], as well as of the Flaviviridae Yellow Fever, West Nile virus, Zika virus and HCV is inhibited by MPA[5,14,15]. The antiviral action of MPA against HCV is partially dependent on the inhibition of IMPDH, but also on the increased expression of antiviral interferon stimulated genes (ISGs) caused by MPA[16].
Until recently, the standard therapy for recurrent HCV infection after transplantation was the combination of pegylated interferon alpha and ribavirin. However, the sustained virological response (SVR) rates were limited between 17% to 45%[17]. The development of direct acting antivirals (DAAs) has led to profound changes in the treatment of HCV. Since 2013, several new generation DAAs have been approved for the treatment of HCV. These include the pan-genotypic NS5A inhibitor daclatasvir (DCV) and the NS3/4A protease inhibitor asunaprevir (ASV)[18,19]. Daclatasvir was approved by the EMA in 2014 and by the FDA in 2015 for treatment of HCV infected individuals. Both drugs were approved by the Japanese Ministry of Health for the treatment of HCV in July 2014. The combination of DCV and ASV was the first combination of DAAs approved for use in Korea in 2015, and in 2017 the combination of DCV and ASV was approved for the treatment of HCV genotype 1 in China[20,21]. The prevalence of HCV infection in Japan, Korea and China is 1.3%, 1.5% and 0.8% respectively, affecting the lives of millions of people[22]. In 2017, a Japanese multicenter study was published about the use of ASV and DSV for recurrence of HCV after liver transplantation, where an SVR12 rate of 80.3% was achieved[23]. According to the authors this SVR rate was unsatisfactory, and indeed in other patient studies in the pre-transplant setting higher SVR rates were reported[21,24,25]. A meta-analysis of 41 studies showed a pooled SVR rate of 89.9% for HCV genotype 1[26]. Although some drug-drug interactions were reported on the pharmacokinetics of DAAs and immunosuppressants[27-32], the potential interference of immunosuppressants with the antiviral activity of DAAs post-transplantation is largely unknown. The aim of our study is to investigate the antiviral action of DCV and ASV in the presence of several different classes of immunosuppressants, using in vitro model systems for HCV replication.
Daclatasvir (DCV) and asunaprevir (ASV) were kindly provided by Bristol-Meyers Squibb (New York, NY, United States). MPA and guanosine were obtained from Sigma (Sigma-Aldrich Chemie, Zwijndrecht, the Netherlands). TAC and CSA were from Abcam (Cambridge, MA, United States). RAPA was obtained from Merck (Amsterdam, the Netherlands). Beetle luciferin potassium salt was from Promega (Promega Benelux BV, Leiden, the Netherlands). All cell lines were cultured in DMEM (Lonza Benelux, Breda, the Netherlands), with 10% fetal calf serum (Sigma-Aldrich Chemie), 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin. Huh7-ETluc cells were cultured in the presence of 500 μg/mL G418 (Life Technologies Europe BV, Bleiswijk, the Netherlands).
The human hepatoma cell line Huh7-ETluc, stably transduced with the HCV bi-cistronic replicon (I389/NS3-3V/LucUbiNeo-ET) containing the nonstructural coding sequences of HCV and the luciferase gene, was used as a model for HCV replication[27]. Huh7-ETluc cells were seeded in white walled, clear bottom 96-well plates (Cellstar, Greiner Bio-one, Alphen a/d Rijn, the Netherlands) at a density of 50000-100000 cells per well. After 16 h the compounds were added in triplicate wells. Cells incubated with vehicle (DMSO) were used as a control. DCV (0.001, 0.01 and 0.1 nmol/L) and ASV (0.1, 1 and 10 nmol/L) were combined with rapamycin (10, 100 and 1000 nmol/L), tacrolimus (0.1, 0.5 and 5.0 μg/mL), cyclosporine A (0.1, 0.5 and 5.0 μg/mL) or MPA (0.1, 0.5 and 5.0 μg/mL). Guanosine (50 μmol/mL) was added to cultures with 0.1 nmol/L DCV and 10 nmol/L ASV in the presence or absence of 5.0 μg/mL MPA to investigate the involvement of the IMPDH pathway on the antiviral action of these compounds. After 24 h luciferase activity was measured. 10 mmol/L Beetle luciferin was added to the cultures and after 30 min luminescence was measured using a Lumistar Optima luminometer. The HCV luciferase activity was calculated as a percentage of the control wells. Huh7 cells stably transduced with a lentiviral vector continuously expressing firefly luciferase (Huh7-PGK-luc) were used as a control to assess non-specific effects of the compounds on luciferase activity and cell growth.
Huh7 cells harboring the full-length JFH-1 derived viral genome were used as an infectious HCV model[28]. 24h after infection the cells were treated with DCV (0.01 and 0.1 nmol/L) and ASV (1 and 10 nmol/L), in combination with 0.5 μg/mL CSA, 5 μg/mL MPA or 5 μg/mL MPA with 50 μmol/mL guanosine. After 48h the cells were lysed, RNA was isolated (Macherey-Nagel Nucleospin RNA kit, Bioké, Leiden, the Netherlands) and quantified using a Nanodrop ND-1000 (Wilmington, DE, United States). cDNA was synthesized using the Primescript RT Master Mix from Takara (Westburg, Leusden, the Netherlands). The levels of HCV-IRES, with GAPDH as a reference gene, were quantified by Reverse Transcription quantitative Polymerase Chain Reaction (RT-qPCR) method using SYBR green (SYBR Select Master Mix, Life Technologies). The relative expression of HCV-IRES (normalized for GAPDH) was calculated as a percentage of the HCV expression in cells that were treated with vehicle only.
Naïve Huh7 cells were cultured in the presence of 5 μg/mL MPA in combination with 0.1 nmol/L DCV or 10 nmol/L ASV. DMSO was used as a vehicle control. After 48 h RNA was isolated and quantified and cDNA was synthesized. The levels of Interferon regulatory factor 1 (IRF1), Interferon regulatory factor 9 (IRF9), and Interferon-induced transmembrane protein 3 (IFITM3), with GAPDH as a reference gene, were quantified with RT-qPCR using SYBR green.
RT-qPCR was performed using the StepOnePlus Real-Time PCR System from Applied Biosystems (Fisher Scientific, Landsmeer, the Netherlands). All reactions were performed in duplicate, 40 cycles of 15’ at 95 °C, 15’ at 58 °C and 1 min at 72 °C, followed by a meltcurve. Primer sequences: IRF1 forward 5-TGCCTCCTGGGAAGATG-3, reverse 5-CCTGGGATTGGTGTTATG-3, IRF9 forward 5-CAAGTGGAGAGTGGGCAGTT-3, reverse 5-ATGGCATCCTCTTCCTCCTT-3, IFITM3 forward 5-CTGGGCTTCATAGCATTCGCCT-3, reverse 5-AGATGTTCAGGCACTTGGCGG-3, IRES forward 5-GTCTAGCCATGGCGTTAGTATGAG-3, reverse 5-ACCCTATCGGCAGACCACAAG-3, GAPDH forward 5-AGAAGGCTGGGGCTCATTTG-3, reverse 5-AGGGGCCATCCACAGTCTTC-3.
All luciferase assays were performed in triplicate and repeated in at least three independent experiments. RT-qPCR analyses were performed in duplicate and repeated in at least two independent experiments. Statistical analysis was performed using GraphPad Prism version 5.01 (Graphpad Software, Inc., La Jolla, California, United States). All data are presented as a mean ± SE. We used a non-parametric Mann-Whitney test (two-tailed, 95%CI) to evaluate the significance of our date. A P-value < 0.05 was considered statistically significant.
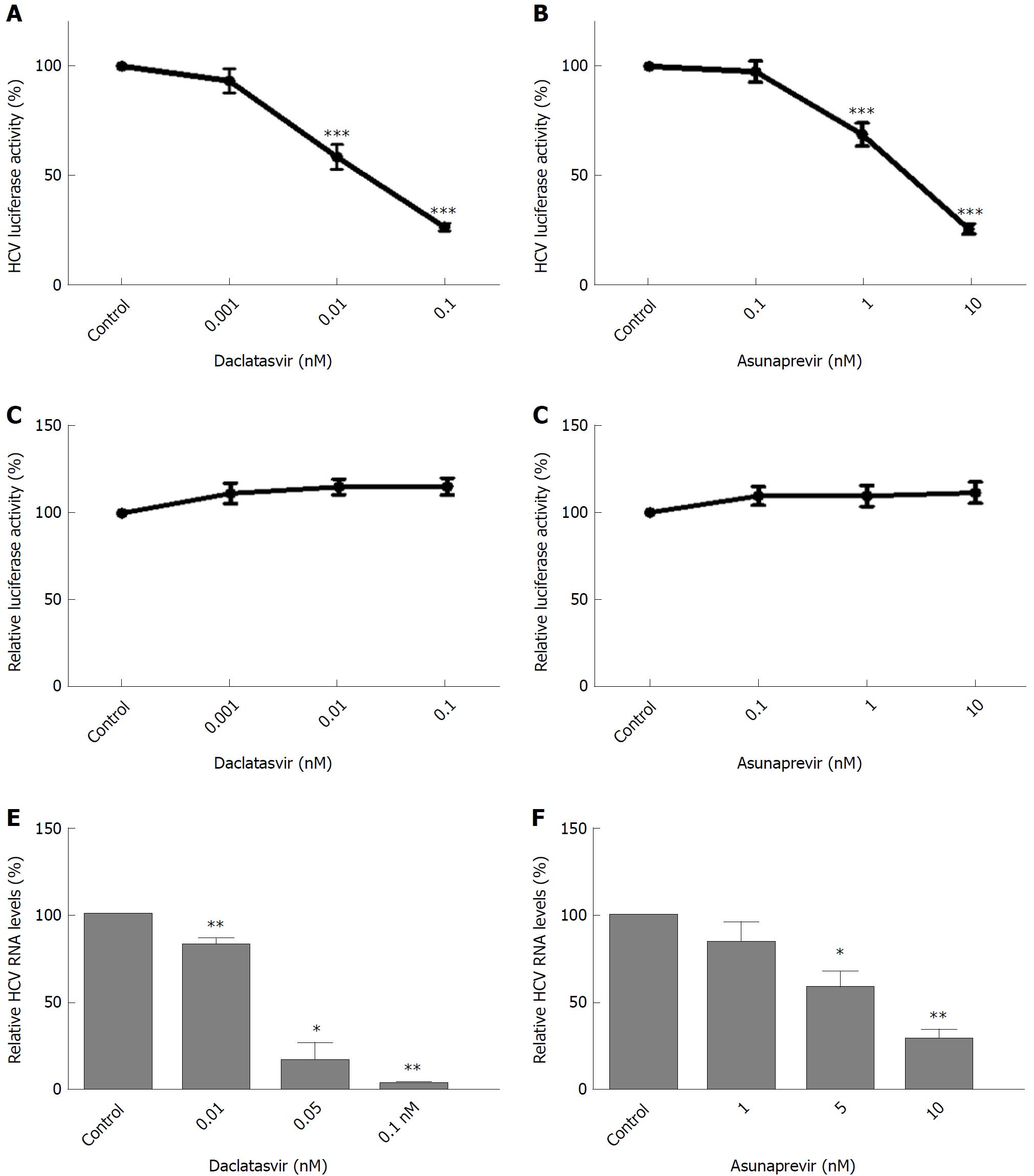
Huh7-ETluc cells were cultured in the presence of different doses of daclatasvir (DCV) and asunaprevir (ASV) and after 24h treatment, HCV replication was measured as luciferase counts. Both DCV and ASV caused a 75% inhibition of HCV replication compared to control levels (Figure 1A and B, P < 0.001). The inhibition of luciferase in Huh7-ETluc cells cannot be attributed to effects of ASV or DCV on cell growth or luciferase activity: when Huh7-PGK-luc cells that stably express luciferase were cultured with ASV or DCV, no inhibition of the luciferase signal could be observed, confirming that the decrease in luciferase signal in Huh7-ETluc cells by DCV and ASV is caused by inhibition of HCV replication (Figure 1C and D). Also in the JFH-derived infectious HCV model, DCV and ASV effectively inhibited HCV replication, with almost complete inhibition by 0.1 nM DCV (Figure 1E, P = 0.004 for 0.01 nM DCV, P = 0.011 for 0.05 nmol/L DVC, P = 0.007 for 0.1 nmol/L DCV), and a 78% reduction compared to control levels by 10nM ASV (Figure 1E and F, P = 0.01 for 5 nmol/L ASV and P = 0.007 for 10 nmol/L ASV).
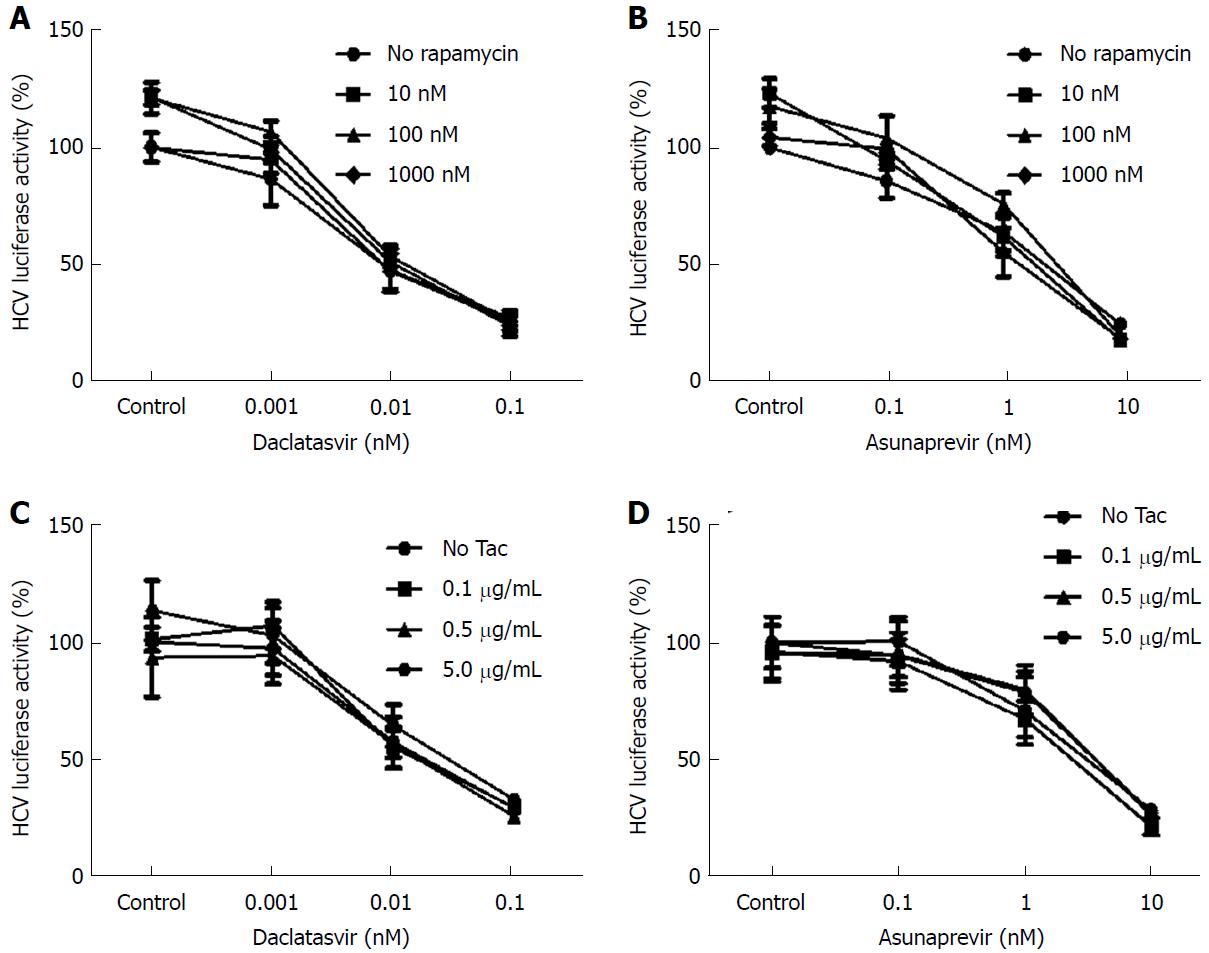
Huh7-ETluc cells were cultured in the presence of different doses of DCV and ASV, in combination with 10, 100 or 1000 nmol/L rapamycin (RAPA). After 24 h of culture HCV replication was measured as luciferase counts. RAPA itself had no effect on viral replication, and the antiviral action of both DCV and ASV was not affected by the addition of RAPA (Figure 2A and B).
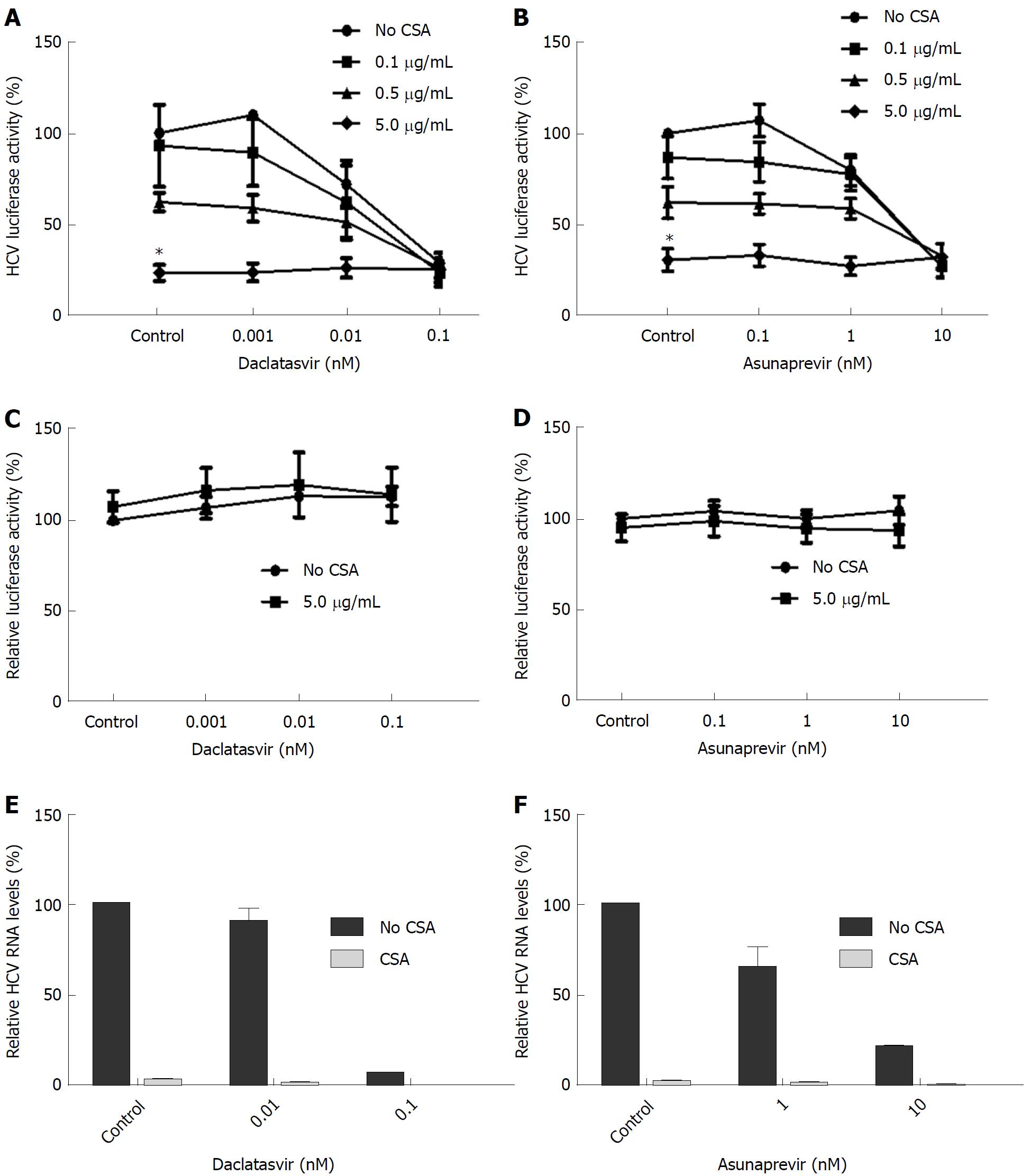
We investigated the effects of the calcineurin inhibitors tacrolimus (TAC) and cyclosporine A (CSA) on the antiviral activity of DCV and ASV. As shown in Figure 2C and 2D, the antiviral action of DCV and ASV was not affected by TAC. As shown in Figure 3A and 3B, contrary to TAC, 5 μg/mL CSA significantly inhibited HCV replication by maximal 76% of control levels (P = 0.03 with DCV, P = 0.04 with ASV).
When combined, the antiviral activity of ASV and DCV was not negatively affected by the addition of CSA. The observed antiviral action of CSA, ASV or DCV in Huh7-ETluc cells cannot be attributed to effects on cell growth or nonspecific effects on luciferase activity. When Huh7-PGK-luc cells were cultured in the presence of ASV or DCV combined with CSA, there was no effect on the luciferase signal (Figure 3C and 3D). In the infectious HCV model, comparable results were found. We observed that HCV replication was inhibited by both ASV and DCV. The addition of 0.5 μg/mL CSA completely inhibited HCV replication at the RNA level and did not negatively affect the inhibition of HCV replication by DCV and ASV (Figure 3E and 3F).
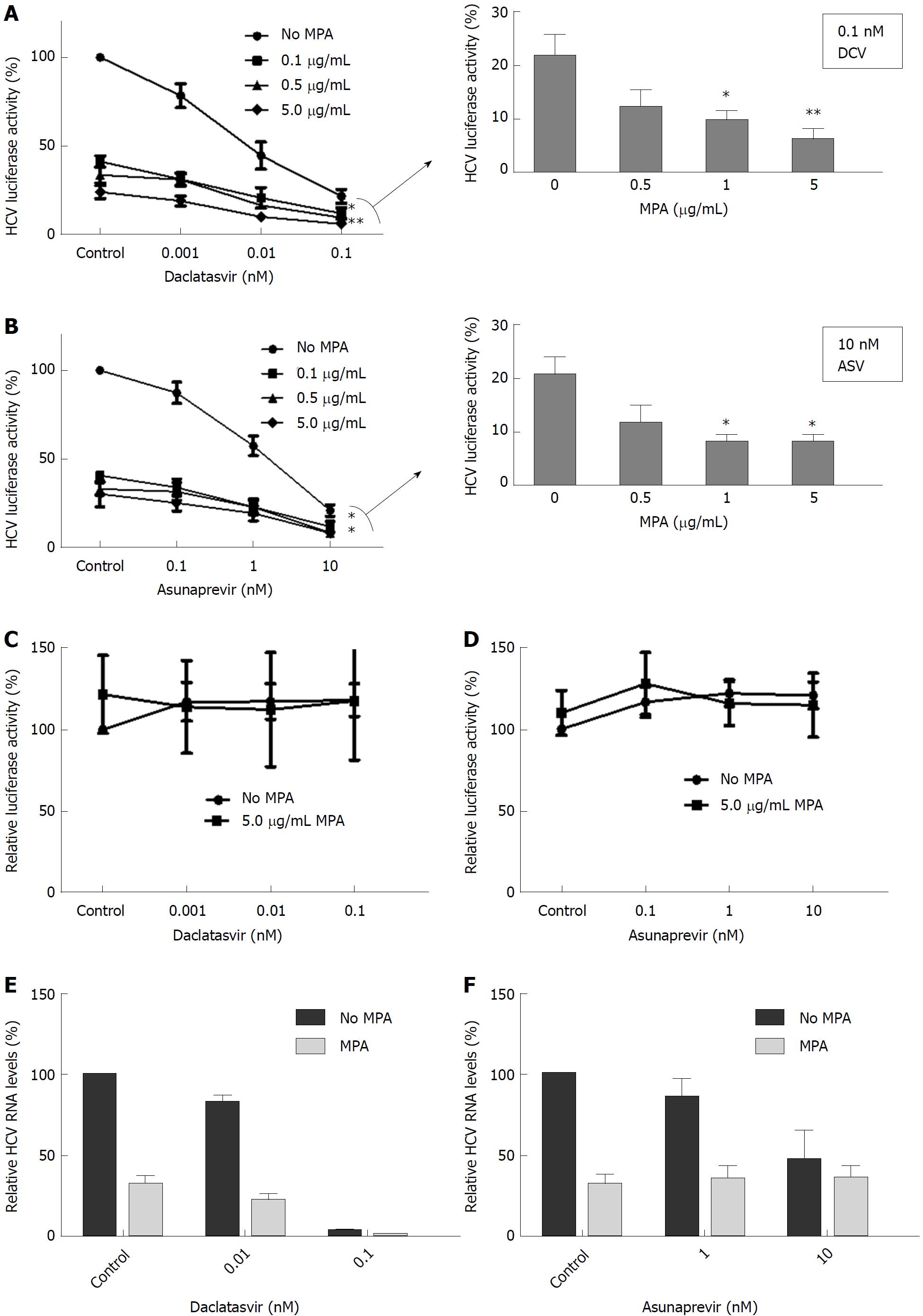
MPA is an immunosuppressant that also affects HCV replication in in vitro cell culture systems. In Huh7-ETluc cells, the addition of MPA resulted in a 70%-76% inhibition of HCV replication compared to control levels. MPA provided additive antiviral effects when combined with ASV or DCV, resulting in an extra inhibition of HCV replication. At the highest doses of DCV and ASV, 1 and 5 μg/mL MPA significantly further decreased HCV replication by an extra 12%-16% (DCV) or 12% (ASV) (Figures 4A and B, P = 0.02 for 1 μg/mL and P = 0.08 for 5 μg/mL MPA with 0.1 nmol/L DCV; P = 0.01 for 1 μg/mL and 5 μg/mL MPA with 10 nmol/L ASV). To investigate if the combined effect of MPA and DAAs on the replication of HCV was not due to non-specific inhibition of luciferase or effects on cell viability, Huh7-PGK cells were cultured with ASV or DCV combined with MPA. The expression of luciferase was not significantly affected by treatment with ASV, DCV or MPA (Figures 4C and D).
In the Huh7 infectious model, 5 μg/mL MPA inhibited HCV replication by 68% of control levels. MPA further inhibited the inhibition of HCV replication by DCV. The highest dose of DCV (0.1 nmol/L) inhibited HCV replication by 96.5% of control levels with an extra reduction by 99.4% of control by MPA (Figure 4E). ASV was less effective in the Huh7 infectious model: when cells were cultured with 10 nmol/L ASV, HCV replication was inhibited by 54% of control levels, and the addition of MPA did not lead to an extra inhibition of HCV replication (Figure 4F).
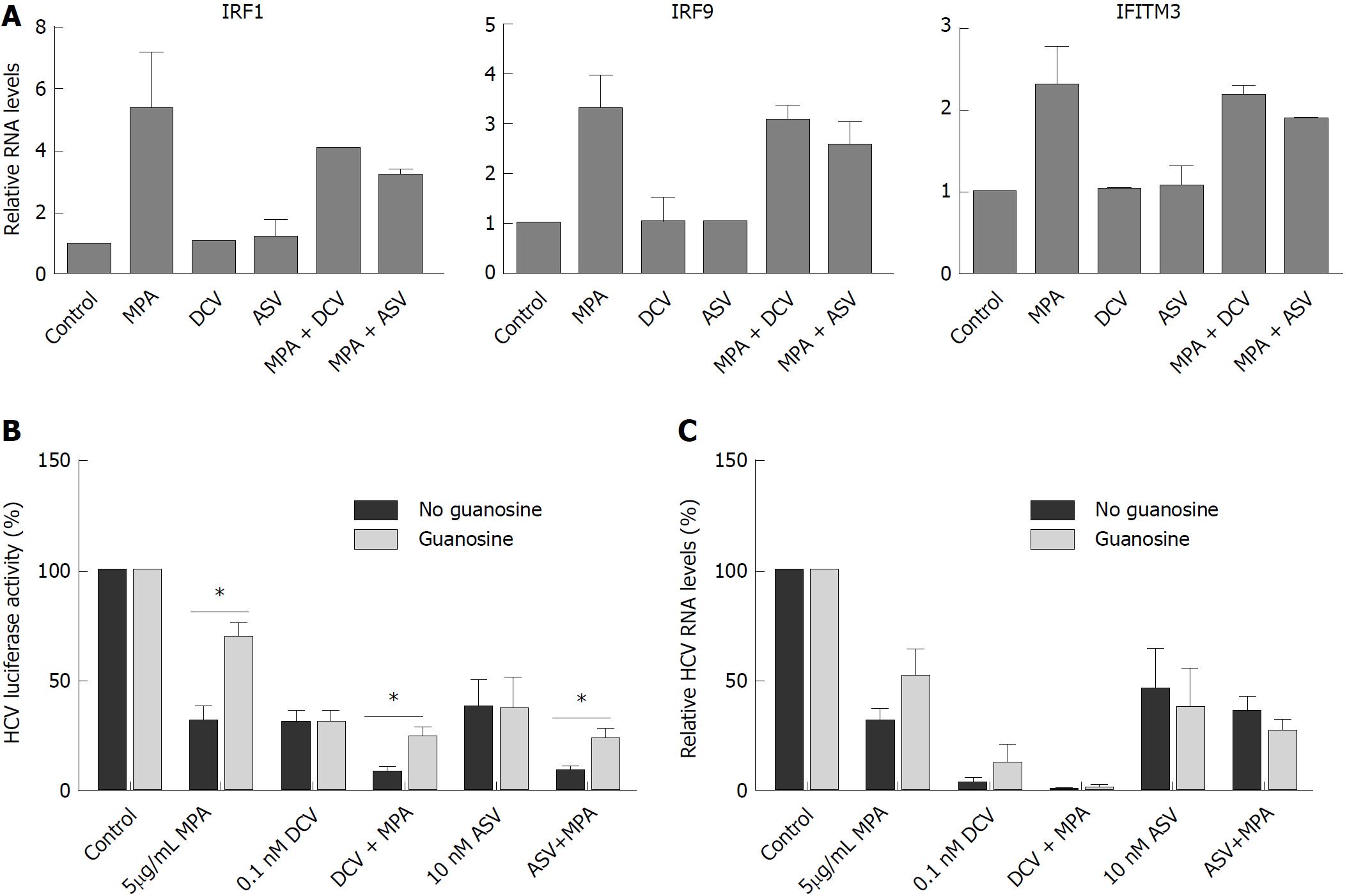
From our previous research, it is known that the antiviral effect of MPA is partially exerted via upregulation of antiviral ISGs[16]. DCV and ASV show a combined antiviral effect with MPA, so we investigated whether the expression of antiviral ISGs was enhanced by the addition of DCV or ASV. Naïve Huh7 cells were cultured for 48 h in the presence of MPA with or without DCV or ASV. After 48 h, total RNA was isolated and the expression of Interferon regulatory factor 1 (IRF1), Interferon regulatory factor 9 (IRF9), and Interferon-induced transmembrane protein 3 (IFITM3) was measured by RT-qPCR. GAPDH was used as a reference gene. The expression of IRF1, IRF9, and IFITM3 was upregulated by 5 μg/mL MPA, but ASV and DCV did not affect the expression of these ISGs, either in the absence or presence of MPA (Figure 5A).
Part of the antiviral effect of MPA on HCV is exerted via inhibition of IMPDH, and subsequent inhibition of guanosine nucleotide biosynthesis. Supplementation with exogenous guanosine can partly reverse the antiviral action of MPA[16]. Therefore, we investigated the role of guanosine supplementation on the antiviral action of DCV or ASV in combination with MPA. As shown in Figure 5B, the addition of 50 μmol/ml guanosine indeed partially reversed the antiviral action of MPA from 69% inhibition to 30% inhibition compared to control levels in Huh7-ETluc cells (P = 0.03) but did not affect the action of DCV or ASV. The combined antiviral effect of MPA and DCV or ASV could significantly be reversed by the addition of guanosine (Figure 5B, P = 0.03 for DSV + MPA and P = 0.03 for ASV + MPA)
We also investigated the effect of guanosine supplementation on the antiviral action of MPA, DCV and ASV in the JFH derived infectious model. After infection, the cells were cultured with DCV or ASV in combination with MPA with or without guanosine. After 48 h, HCV RNA levels were determined by RT-qPCR. MPA inhibited HCV replication by 68% of control levels. This could be partly (but not significantly) reversed to 49% inhibition compared to control levels by the addition of guanosine. DCV (0.1 nmol/L) inhibited HCV replication by 96.5% of control levels, with no significant effect of guanosine. The addition of MPA further reduced HCV replication to more than 99% of control levels, however with no effect of guanosine supplementation. 10 nmol/L ASV reduced HCV replication by 54% of control levels, with no additional effect of MPA. The addition of guanosine also had no effect on the inhibition of HCV replication by ASV, either in the presence or absence of MPA (Figure 5C).
The potential interference of immunosuppressants with the antiviral activity of DAAs post-transplantation is largely unknown. In 2017, Ikegami et al[23] showed in their study that the SVR rate of 80.3% that was achieved in patients who were treated with DCV and ASV after transplantation was not satisfactory. We aimed to investigate the interaction between immunosuppressants and DCV and ASV, both newer generation DAAs for the treatment of HCV. In our two in vitro HCV culture models, the mTOR inhibitor rapamycin and the calcineurin inhibitor tacrolimus did not negatively affect the antiviral action of DCV and ASV.
The calcineurin inhibitor CSA inhibited HCV replication, as described previously[6,10]. The addition of CSA did not negatively affect the antiviral action of DCV and ASV. The CSA concentrations we used in our study (between 100 and 5000 ng/mL) are in a clinically relevant range. Cyclosporine A target levels in patients range between 700-1300 ng/mL measured in blood[33], and peak levels vary between 800-2285 ng/mL[34]. In liver tissue, CSA levels can be 2.7 times higher as compared to plasma levels[35].
MPA, like CSA, inhibited HCV replication in vitro. The concentrations of MPA we used (0.1-5 μg/mL) are clinically achievable. In patients receiving MMF or MPA, serum peak levels range from 0.6 to11.5 μg/mL and trough levels average around 3 μg/mL[36]. Animal studies have shown that MPA accumulates in the liver[37]. When DCV and ASV were combined with MPA in our experiments, there was a difference in effect on the antiviral action compared to the experiments with CSA. When MPA was combined with the highest concentrations of DCV and ASV, an extra inhibition of HCV replication was observed, that could not be achieved with DCV or ASV alone. The combined antiviral effect was also observed in an infectious HCV model, but only with MPA and DCV. MPA exerts its antiviral action on HCV via two pathways: through the induction of antiviral ISGs and via inhibition of IMPDH, leading to depletion of the GTP pool in the cell. We did not observe upregulation of antiviral ISGs in cells that were cultured with DCV or ASV, and the upregulation of ISGs by MPA was not affected by the addition of these DAAs. In Huh7-ETluc cells, supplementation of the GTP pool by guanosine partly reversed the antiviral effect of MPA, and also the combined antiviral action of DCV or ASV with MPA. However, in the infectious model, only the antiviral activity of MPA was (partly) reversed by guanosine, and not the combined antiviral action of MPA and DCV. These results indicate that the inhibition of GTP synthesis by MPA is (partly) involved in the combined antiviral action of MPA with DSV and ASV. The difference in responsiveness to DCV or ASV we observe between Huh7-ETluc cells and the JFH infectious model might be explained by the fact that DCV is a pan-genotypic HCV inhibitor, while ASV is more specific for genotype 1b and is less active against genotypes 2 and 3[38,39]. The genotype of HCV in the JFH infectious model is 2a and the HCV construct in the Huh7-ETluc cells is derived from genotype 1b.
Although the in vitro antiviral action of MPA has been well documented, the clinical effects of MPA on HCV replication remain controversial. Some patient studies showed a significant reduction of HCV viral load by MMF treatment[40,41], while others reported no effects on HCV infection[42-44]. Ikegami et al[23] show in their study that 46.9% of patients who achieved SVR were treated with MMF, whereas 38.4% of the no-SVR group received MMF. However, this putative positive effect of MMF on DAA-induced SVR was not significant[23].
Our in vitro study shows that none of the immunosuppressants we tested negatively interfered with the antiviral action of DSV and ASV. The combination of MPA with DSV and ASV resulted in a higher reduction of HCV replication than that could be achieved by treatment with these compounds alone. Although the antiviral action of MPA is evident in cell culture systems, the antiviral effect in patients might be masked by the suppressive effects of MPA on the immune response. Our results can, however, complement the still emerging clinical findings on the effectivity of DAAs in the presence of immunosuppressants. Based on this in vitro study, there is no rationale or evidence to withhold or adjust DCV or ASV in combination with immunosuppressants in the post-transplantation management of HCV.
Liver disease caused by chronic Hepatitis C virus (HCV) infection is a leading indication for liver transplantation. Factors that contribute to the recurrence of HCV after transplantation include viral factors (e.g., HCV RNA levels at the time of transplantation and HCV genotype), host factors (immune response and HCV cryoglobulinemia), and the use of immunosuppressive medication. Current treatment of HCV is based on direct acting antivirals (DAAs), including daclatasvir (DCV) and asunaprevir (ASV). Recently a study reported reduced sustained virological response rates with DCV/ASV therapy after transplantation, indicating potential interference with immunosuppressants.
Although some drug-drug interactions were reported on the pharmacokinetics of DAAs and immunosuppressants, the potential interference of immunosuppressants with the antiviral activity of DAAs post-transplantation is largely unknown.
The aim of our study is to investigate the antiviral action of DCV and ASV in the presence of several different classes of immunosuppressants.
The antiviral activity of DCV and ASV combined with immunosuppressants was tested using two in vitro cell culture models for HCV infection. The cells were cultured with different concentrations of DCV or ASV in combination with immunosuppressants from several different classes. The effects on HCV replication were quantified by luciferase assay or quantitative RT-PCR. Effects on the expression of antiviral interferon-stimulated genes were also assessed by quantitative RT-PCR.
Tacrolimus, rapamycin and cyclosporine did not negatively affect the antiviral action of DCV or ASV. Mycophenolic acid (MPA) showed additive antiviral effects combined with these DAAs. MPA induces interferon-stimulated genes (ISGs) and is a potent GTP synthesis inhibitor. DCV or ASV did not induce expression of ISGs nor affected ISG induction by MPA. Rather, the combined antiviral effect of MPA with DCV and ASV was partly mediated via inhibition of GTP synthesis.
Our in vitro study shows that none of the immunosuppressants we tested negatively interfered with the antiviral action of DSV and ASV. The combination of MPA with DSV and ASV resulted in a higher reduction of HCV replication than that could be achieved by treatment with these compounds alone. Although the antiviral action of MPA is evident in cell culture systems, the antiviral effect in patients might be masked by the suppressive effects of MPA on the immune response. Our results can, however, complement the still emerging clinical findings on the effectivity of DAAs in the presence of immunosuppressants.
Based on this in vitro study, there is no rationale or evidence to withhold or adjust DCV or ASV in combination with immunosuppressants in the post-transplantation management of HCV.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bredt lc, Mukherjee S, Uhlmann D, Yang SS S- Editor: Ma YJ L- Editor: A E- Editor: Yin SY
| 1. | Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14 Suppl 2:S36-S44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Henry SD, Metselaar HJ, Van Dijck J, Tilanus HW, Van Der Laan LJ. Impact of steroids on hepatitis C virus replication in vivo and in vitro. Ann N Y Acad Sci. 2007;1110:439-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | de Ruiter PE, Boor PP, de Jonge J, Metselaar HJ, Tilanus HW, Ijzermans JN, Kwekkeboom J, van der Laan LJ. Prednisolone does not affect direct-acting antivirals against hepatitis C, but inhibits interferon-alpha production by plasmacytoid dendritic cells. Transpl Infect Dis. 2015;17:707-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Perry I, Neuberger J. Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol. 2005;139:2-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Henry SD, Metselaar HJ, Lonsdale RC, Kok A, Haagmans BL, Tilanus HW, van der Laan LJ. Mycophenolic acid inhibits hepatitis C virus replication and acts in synergy with cyclosporin A and interferon-alpha. Gastroenterology. 2006;131:1452-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Nakagawa M, Sakamoto N, Enomoto N, Tanabe Y, Kanazawa N, Koyama T, Kurosaki M, Maekawa S, Yamashiro T, Chen CH. Specific inhibition of hepatitis C virus replication by cyclosporin A. Biochem Biophys Res Commun. 2004;313:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282-1288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 402] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 8. | Ma C, Li F, Musharrafieh RG, Wang J. Discovery of cyclosporine A and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance. Antiviral Res. 2016;133:62-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Pan Q, Tilanus HW, Metselaar HJ, Janssen HL, van der Laan LJ. Virus-drug interactions--molecular insight into immunosuppression and HCV. Nat Rev Gastroenterol Hepatol. 2012;9:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Pan Q, Metselaar HJ, de Ruiter P, Kwekkeboom J, Tilanus HW, Janssen HL, van der Laan LJ. Calcineurin inhibitor tacrolimus does not interfere with the suppression of hepatitis C virus infection by interferon-alpha. Liver Transpl. 2010;16:520-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Yin Y, Wang Y, Dang W, Xu L, Su J, Zhou X, Wang W, Felczak K, van der Laan LJ, Pankiewicz KW. Mycophenolic acid potently inhibits rotavirus infection with a high barrier to resistance development. Antiviral Res. 2016;133:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | To KK, Mok KY, Chan AS, Cheung NN, Wang P, Lui YM, Chan JF, Chen H, Chan KH, Kao RY. Mycophenolic acid, an immunomodulator, has potent and broad-spectrum in vitro antiviral activity against pandemic, seasonal and avian influenza viruses affecting humans. J Gen Virol. 2016;97:1807-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Zhou X, Debing Y, Chen K, Van Der Laan LJ, Neyts J, Janssen HL, Metselaar HJ, Peppelenbosch MP, Pan Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology. 2014;146:1775-1783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Diamond MS, Zachariah M, Harris E. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology. 2002;304:211-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, Galarza-Muñoz G, McGrath EL, Urrabaz-Garza R, Gao J. A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe. 2016;20:259-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 16. | Pan Q, de Ruiter PE, Metselaar HJ, Kwekkeboom J, de Jonge J, Tilanus HW, Janssen HL, van der Laan LJ. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55:1673-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl. 2006;12:1192-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Belema M, Meanwell NA. Discovery of daclatasvir, a pan-genotypic hepatitis C virus NS5A replication complex inhibitor with potent clinical effect. J Med Chem. 2014;57:5057-5071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Scola PM, Sun LQ, Wang AX, Chen J, Sin N, Venables BL, Sit SY, Chen Y, Cocuzza A, Bilder DM. The discovery of asunaprevir (BMS-650032), an orally efficacious NS3 protease inhibitor for the treatment of hepatitis C virus infection. J Med Chem. 2014;57:1730-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Bristol-Myers Squibb. 2017. Available from: https://www.bms.com/cn. [Cited in This Article: ] |
| 21. | Cho BW, Kim SB, Song IH, Lee SH, Kim HS, Lee TH, Kang YW, Kim SH, Lee BS, Chae HB. Efficacy and safety of daclatasvir plus asunaprevir for Korean patients with HCV genotype Ib infection: a retrospective multi-institutional study. Clin Mol Hepatol. 2017;23:51-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824-7840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 514] [Cited by in F6Publishing: 507] [Article Influence: 63.4] [Reference Citation Analysis (7)] |
| 23. | Ikegami T, Ueda Y, Akamatsu N, Ishiyama K, Goto R, Soyama A, Kuramitsu K, Honda M, Shinoda M, Yoshizumi T. Asunaprevir and daclatasvir for recurrent hepatitis C after liver transplantation: A Japanese multicenter experience. Clin Transplant. 2017;31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Ishigami M, Hayashi K, Honda T, Kuzuya T, Ishizu Y, Ishikawa T, Nakano I, Urano F, Kumada T, Yoshioka K. Daclatasvir and asunaprevir treatment in patients infected by genotype 1b of hepatitis C virus with no or subtle resistant associated substitutions (RAS) in NS5A-Y93. J Med Virol. 2018;90:736-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Kao JH, Lee YJ, Heo J, Ahn SH, Lim YS, Peng CY, Chang TT, Torbeyns A, Hughes E, Bhore R. All-oral daclatasvir plus asunaprevir for chronic hepatitis C virus (HCV) genotype 1b infection: a sub-analysis in Asian patients from the HALLMARK DUAL study. Liver Int. 2016;36:1433-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Ji F, Wei B, Yeo YH, Ogawa E, Zou B, Stave CD, Li Z, Dang S, Furusyo N, Cheung RC. Systematic review with meta-analysis: effectiveness and tolerability of interferon-free direct-acting antiviral regimens for chronic hepatitis C genotype 1 in routine clinical practice in Asia. Aliment Pharmacol Ther. 2018;47:550-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Lohmann V, Hoffmann S, Herian U, Penin F, Bartenschlager R. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J Virol. 2003;77:3007-3019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 28. | Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2241] [Cited by in F6Publishing: 2239] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 29. | Bifano M, Adamczyk R, Hwang C, Kandoussi H, Marion A, Bertz RJ. An open-label investigation into drug-drug interactions between multiple doses of daclatasvir and single-dose cyclosporine or tacrolimus in healthy subjects. Clin Drug Investig. 2015;35:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Herzer K, Papadopoulos-Köhn A, Walker A, Achterfeld A, Paul A, Canbay A, Timm J, Gerken G. Daclatasvir, Simeprevir and Ribavirin as a Promising Interferon-Free Triple Regimen for HCV Recurrence after Liver Transplant. Digestion. 2015;91:326-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Nakamura Y, Imamura M, Kawakami Y, Teraoka Y, Daijo K, Honda F, Morio K, Kobayashi T, Nakahara T, Nagaoki Y. Efficacy and safety of daclatasvir plus asunaprevir therapy for chronic hepatitis C patients with renal dysfunction. J Med Virol. 2017;89:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Fernández I, Muñoz-Gómez R, Pascasio JM, Baliellas C, Polanco N, Esforzado N, Arias A, Prieto M, Castells L, Cuervas-Mons V. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol. 2017;66:718-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 33. | Kahn D, Mazzaferro V, Cervio G, Venkataramanan R, Makowka L, Van Thiel DH, Starzl TE. Correlation between dose and level of cyclosporine after orthotopic liver transplantation. Transplant Proc. 1989;21:2240-2241. [PubMed] [Cited in This Article: ] |
| 34. | Halloran PF, Helms LM, Kung L, Noujaim J. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation. 1999;68:1356-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 265] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 35. | Kahan BD, Van Buren CT, Boileau M, Ried M, Payne WD, Flechner S, Newburger J. Cyclosporin A tissue levels in a cadaveric renal allograft recipient. Transplantation. 1983;35:96-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Patel CG, Akhlaghi F. High-performance liquid chromatography method for the determination of mycophenolic acid and its acyl and phenol glucuronide metabolites in human plasma. Ther Drug Monit. 2006;28:116-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Matsuzawa Y, Nakase T. Metabolic fate of ethyl O-[N-(p-carboxyphenyl)-carbamoyl] mycophenolate (CAM), a new antitumor agent, in experimental animals. J Pharmacobiodyn. 1984;7:776-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3:514-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Gentile I, Buonomo AR, Zappulo E, Minei G, Morisco F, Borrelli F, Coppola N, Borgia G. Asunaprevir, a protease inhibitor for the treatment of hepatitis C infection. Ther Clin Risk Manag. 2014;10:493-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Kornberg A, Küpper B, Tannapfel A, Hommann M, Scheele J. Impact of mycophenolate mofetil versus azathioprine on early recurrence of hepatitis C after liver transplantation. Int Immunopharmacol. 2005;5:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Bahra M, Neumann UI, Jacob D, Puhl G, Klupp J, Langrehr JM, Berg T, Neuhaus P. MMF and calcineurin taper in recurrent hepatitis C after liver transplantation: impact on histological course. Am J Transplant. 2005;5:406-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Jain A, Kashyap R, Demetris AJ, Eghstesad B, Pokharna R, Fung JJ. A prospective randomized trial of mycophenolate mofetil in liver transplant recipients with hepatitis C. Liver Transpl. 2002;8:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, McMaster P, Kalayoglu M, Levy G, Freeman R. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001;7:442-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Fasola CG, Netto GJ, Jennings LW, Christensen LL, Molmenti EP, Sanchez EQ, Levy MF, Goldstein RM, Klintmalm GB. Recurrence of hepatitis C in liver transplant recipients treated with mycophenolate mofetil. Transplant Proc. 2002;34:1563-1564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |