Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.231
Peer-review started: June 27, 2015
First decision: August 26, 2015
Revised: September 11, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: December 24, 2015
Processing time: 180 Days and 18 Hours
After successful kidney transplantation, accumulated waste products and electrolytes are excreted and regulatory hormones return to normal levels. Despite the improvement in mineral metabolites and mineral regulating hormones after kidney transplantation, abnormal bone and mineral metabolism continues to present in most patients. During the first 3 mo, fibroblast growth factor-23 (FGF-23) and parathyroid hormone levels decrease rapidly in association with an increase in 1,25-dihydroxyvitamin D production. Renal phosphate excretion resumes and serum calcium, if elevated before, returns toward normal levels. FGF-23 excess during the first 3-12 mo results in exaggerated renal phosphate loss and hypophosphatemia occurs in some patients. After 1 year, FGF-23 and serum phosphate return to normal levels but persistent hyperparathyroidism remains in some patients. The progression of vascular calcification also attenuates. High dose corticosteroid and persistent hyperparathyroidism are the most important factors influencing abnormal bone and mineral metabolism in long-term kidney transplant (KT) recipients. Bone loss occurs at a highest rate during the first 6-12 mo after transplantation. Measurement of bone mineral density is recommended in patients with estimated glomerular filtration rate > 30 mL/min. The use of active vitamin D with or without bisphosphonate is effective in preventing early post-transplant bone loss. Steroid withdrawal regimen is also beneficial in preservation of bone mass in long-term. Calcimimetic is an alternative therapy to parathyroidectomy in KT recipients with persistent hyperparathyroidism. If parathyroidectomy is required, subtotal to near total parathyroidectomy is recommended. Performing parathyroidectomy during the waiting period prior to transplantation is also preferred in patients with severe hyperparathyroidism associated with hypercalcemia.
Core tip: Despite the improvement in mineral metabolites and mineral regulating hormones after kidney transplantation, abnormal mineral metabolism continues to present in most patients. High dose corticosteroid and persistent hyperparathyroidism are the most important factors influencing abnormal mineral metabolism in long-term kidney transplant recipients. The use of active vitamin D with or without bisphosphonate and steroid withdrawal regimen are effective in preventing early post-transplant bone loss. Calcimimetic is an alternative therapy to parathyroidectomy. If parathyroidectomy is required, subtotal to near total parathyroidectomy is recommended. Performing parathyroidectomy during the waiting period is also preferred in patients with severe hyperparathyroidism associated with hypercalcemia.
- Citation: Taweesedt PT, Disthabanchong S. Mineral and bone disorder after kidney transplantation. World J Transplant 2015; 5(4): 231-242
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/231.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.231
After successful kidney transplantation, kidney function resumes. Accumulated waste products and electrolytes are excreted and regulatory hormones return to normal levels. Important mineral metabolites and regulatory hormones in bone and mineral metabolism include calcium, phosphate, parathyroid hormone (PTH), fibroblast growth factor-23 (FGF-23) and vitamin D. Improvement of bone and mineral metabolism are expected in most patients and, as a result, the progression of vascular calcification also attenuates. However, persistent abnormalities remain in some patients. Due to the dependency on life-long immunosuppression especially corticosteroid, new bone disorder may also develop. This review focuses on abnormalities of bone and mineral metabolism that occur after kidney transplantation.
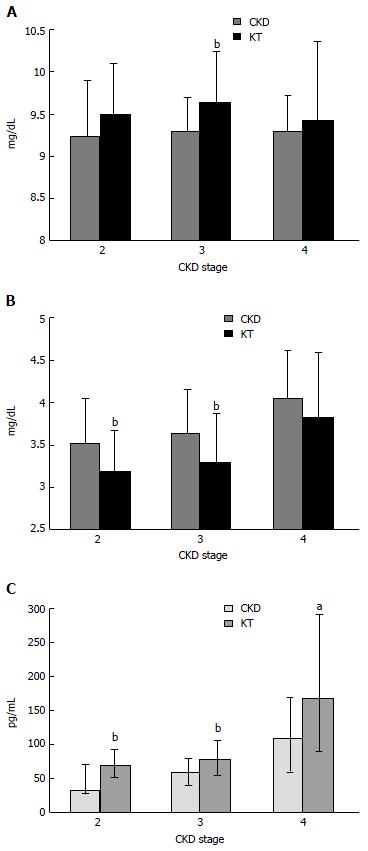
Prior to kidney transplantation, chronic kidney disease (CKD) patients often have high FGF-23 level as a result of phosphate retention. After successful kidney transplantation, as kidney function resumes, urinary phosphate excretion is normally exaggerated by the relatively high FGF-23 concentration resulting in renal phosphate wasting and low serum phosphate in some patients. Despite the rapid reduction of FGF-23 during the first 3 d up until 3 mo after transplantation, the average FGF-23 level is still higher than normal resulting in almost 90% of patients with functioning graft experiencing hypophosphatemia at some point[1-3]. The degree of hypophosphatemia is mild-to-moderate (1.5-2.3 mg/dL) in 20% and severe (≤ 1.5 mg/dL) in 60% of the patients. After 3 mo, FGF-23 levels still elevate in 60% and hypophosphatemia can still be observed in 30%. FGF-23 levels at 3 mo after transplantation are independently associated with fractional excretion of phosphate (FEP), decreased calcitriol levels and pre-transplant FGF-23 levels. There are no correlations between phosphate parameters and PTH during this early period[1,3]. The degree of hypophosphatemia can also be predicted by pre-transplant FGF-23 levels[4]. FGF-23 normally returns to baseline approximately 1 year after transplantation[5,6]. Among patients who have been transplanted for longer than 10 years with a well-functioning graft, FGF-23 levels are comparable to CKD patients matched for estimated glomerular filtration rate (eGFR)[7]. Nevertheless, despite the return of FGF-23 to baseline after 1 year, serum phosphate is still significantly lower than that in CKD patients[5]. Studies on phosphate metabolism in this later period reveal lower serum phosphate and higher serum calcium compared to CKD patients with equivalent eGFR and hypophosphatemia can still be observed in 5%-6% of the patients[8,9] (Figure 1A and B). Low serum phosphate is the result of phosphate loss in the urine but, in contrast to the early period, FGF-23 is not responsible for phosphaturia because FGF-23 levels are lower than the levels observed in CKD patients[9,10]. The presence of decreased serum phosphate and increased serum calcium seems to suggest the role of PTH in renal phosphate loss. In fact, PTH levels in kidney transplant (KT) recipients are higher than that in CKD patients at all levels of kidney function and only increased PTH level displays an independent association with FEP during this later period after kidney transplantation[8] (Figure 1C).
PTH levels decline substantially during the first 3 mo after kidney transplantation. However, a significant number of KT recipients with adequate allograft function still exhibits high PTH levels[11]. In long-term KT recipients with a well-functioning graft (eGFR > 30-45 mL/min), high PTH level can still be observed in 30%-60% one year after transplantation[5,7,8,12]. Elevated PTH level in this later period is responsible for an increase in serum calcium, a decrease in serum phosphate and an increase in FEP suggesting that the secretion of PTH is not entirely under the normal feedback control[8,13]. High PTH level prior to transplantation, long dialysis vintage, and monoclonal transformation (nodular hyperplasia) of parathyroid glands are important risk factors for the persistence of hyperparathyroidism after transplantation. Nodular hyperplastic parathyroid gland exhibits a decrease in calcium sensing receptor (CaSR), vitamin D receptor (VDR) and FGFR1-Klotho expression resulting in an upward increase in the set point of calcium that triggers PTH release and a resistance to active vitamin D and FGF-23[9,11,14-17]. Pre-transplant PTH and calcium levels can also predict the severity of persistent hyperparathyroidism and the need for parathyroid surgery after transplantation[18]. Restoration of CaSR and VDR expression after successful transplantation which can allow the shrinkage of gland size is expected only in non-nodular hyperplastic glands[19]. Due to the long life span of parathyroid cells (approximately 20 years) with a cell renewal rate of only 5% per year, the decrease in PTH level after the first 3 mo occurs at a very slow rate. Therefore, patients with high PTH level prior to transplantation are likely to experience long-term persistent hyperparathyroidism. The use of calcimimetic drug during the waiting period can also influence the degree of hyperparathyroidism after transplantation. In a study that compared patients who had been on cinacalcet during the waiting period and then discontinued after transplantation to those who had never been on the drug revealed a higher incidence of post-transplant nephrocalcinosis and parathyroidectomy in patients who had been on cinacalcet before[20].
25-hydroxyvitamin D (25-OH-D) deficiency is commonly observed in KT recipients. Recent study in the northern latitude of European continent revealed 49% of long-term KT recipients (median transplant vintage of 6 years) were vitamin D deficient (25-OH-D < 20 ng/mL), 33% were insufficient (20-30 ng/mL) and 18% were sufficient (> 30 ng/mL)[21]. Other studies in western countries found similar results with only 10%-20% of patients had sufficient 25-OH-D level[22-25]. Studies in Asian countries closer to the Equator with more sun exposure revealed the prevalence of 25-OH-D deficiency ranging between 20%-30%, 25-OH-D insufficiency around 50% and 25-OH-D sufficiency ranging between 25%-30%[8,26]. Time from transplantation seems to positively influence 25-OH-D level in which every year out of transplantation decreases the risk of deficiency by approximately 10%[27]. In addition to the reduced sunlight exposure, the use of sun protectors, and the impaired kidney function, the use of immunosuppressive drugs especially high doses of steroid, and the presence of metabolic syndrome and obesity are also associated with 25-OH-D deficiency[26,28]; Lower 25-OH-D level in KT recipients can worsen the degree of hyperparathyroidism by depleting the substrate for 1,25-dihydroxyvitamin D (1,25-OH2-D) production[25]. Severe 1,25-OH2-D deficiency can be observed in up to 80% in the immediate post-transplant period[29]. The concentration of 1,25-OH2-D increases rapidly thereafter and becomes comparable to CKD patients with equivalent kidney function after 3-12 mo[5]. During the early period post-transplantation, 1,25-OH2-D levels are negatively correlated with FGF-23 levels suggesting that the excess of FGF-23 suppresses the production of 1,25-OH2-D. Twelve months after transplantation, only allograft function displays an association with 1,25-OH2-D level indicating the return of vitamin D physiology towards that of CKD[5]. Roles of vitamin D in KT patients are diverse. In addition to the effect on bone and mineral metabolism, vitamin D also exerts several important immunological effects. The effect of vitamin D on adaptive immune responses including inhibition of dendritic cell proliferation and maturation causing an impairment of antigen presenting activity may reduce the risk of transplant rejection[30]. In addition to suppression of cell growth, vitamin D also promotes cell apoptosis while inhibiting angiogenesis which may also protect against cancer development after transplantation[31]. Details of this topic can be found in a review by McGregor et al[32].
Immediately after successful kidney transplantation, serum calcium decreases secondary to the discontinuation of calcium and active vitamin D. The rapid decline in PTH results in the movement of calcium back into the bone and the loss of calcium in the urine[33]. Thereafter, serum calcium gradually increases and becomes stabilized after 3-6 mo. Due to the high prevalence of persistent hyperparathyroidism as mentioned earlier, hypercalcemia usually develops in 10%-15% of KT recipients[8,12]. Pre-transplant calcium and PTH levels are the significant determinant of hypercalcemia after transplantation[6,15]. Increased serum calcium may also occur in association with low PTH levels. In this case, other causes such as malignancy and opportunistic infection should be considered. Hypercalcemia in conjunction with pneumocystis jirovecii pneumonia (PCP) is being increasingly reported in immunocompromised patients[34]. The increase in serum calcium was due to granulomatous PCP infection and extrarenal production of 1,25-OH2-D[35]. Hypercalcemia may be a prodromal feature of indolent PCP infection with full blown pneumonia developing few months later[36]. Hypercalcemia and suppressed PTH level normally resolve after a successful treatment of pneumonia.
The prevalence of osteoporosis in long-term KT recipients ranges between 11%-56% with the incidence of vertebral fracture 3%-29% and peripheral fracture 11%-43%[37]. Bone loss occurs at a highest rate in the first 6 mo and continues to occur at a slower rate during the following 6-12 mo after transplantation[38,39]. According to bone mineral density (BMD) data, the rate of bone loss in the first 6 mo ranges between 5.5%-19.5%, which decreases to 2.6%-8.2% after 6-12 mo. After the first year, BMD largely stabilizes but, in some patients, a gradual decline may still be observed at a rate between 0.4%-4.5%[40]. The data on bone histology in KT recipients revealed abnormalities in nearly all patients. The decrease in bone volume and bone formation was observed indicating the presence of adynamic bone disease[41]. In addition, there was an increase in osteoblast apoptosis, some degree of mineralization defect as well as an increase in bone resorption[42]. Abnormal bone pathology and bone loss that occur after kidney transplantation are largely due to the high cumulative dose of corticosteroid and persistent hyperparathyroidism[43]. Corticosteroid can inhibit osteoblastogenesis, suppress bone formation, promote osteoblast apoptosis, stimulate bone resorption and attenuate calcium absorption from the intestine[44]. Persistent hyperparathyroidism is an important factor for the increased bone resorption after kidney transplantation[39]. Despite the presence of hypophosphatemia in the early post-transplant period, only 5% of KT recipients display bone histologic finding consistent with osteomalacia[45]. In addition to corticosteroid and hyperparathyroidism, factors other than age, gender and diabetes that may influence post-transplant bone loss include long dialysis vintage, previous transplantation and poor allograft function[46]. A recently published study also revealed the relationship between hepatitis C virus infection and post-transplant osteoporosis[47]. As for fracture risk, the risk of hip fracture in KT recipients during the first 6 mo after transplantation is 34% higher than that in dialysis patients[48]. In long-term KT recipients, fracture risk within 10 years of transplantation is 4 times higher than fracture risk in general population[49]. After 10 years, the risk decreases to twice of that in general population[50].
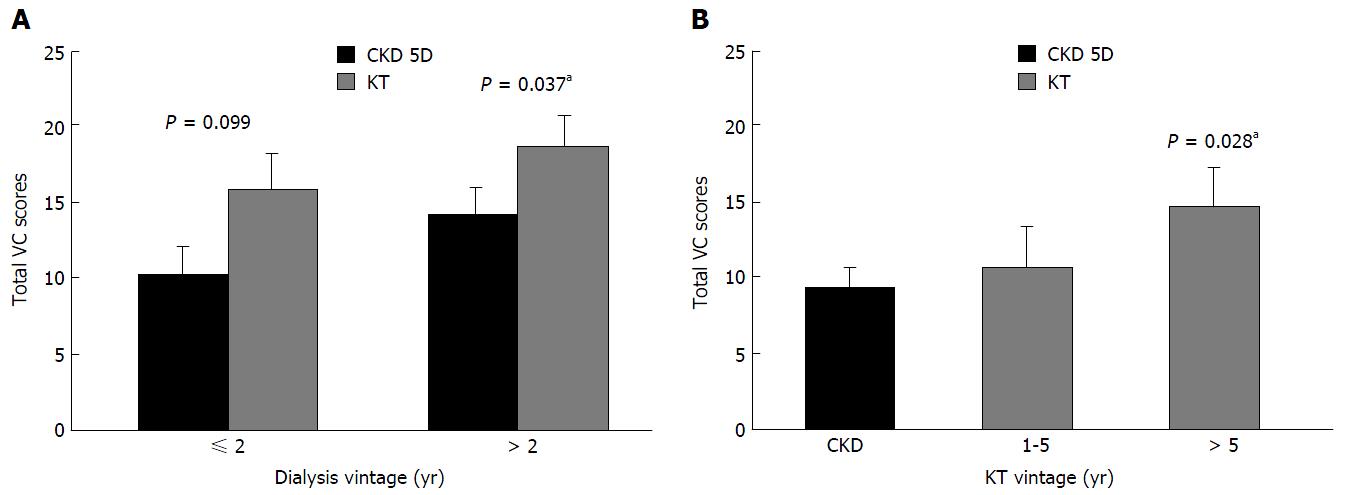
Atherosclerosis and vascular calcification are common among patients with CKD due to the high prevalence of cardiovascular risk factors including aging, smoking, diabetes, hypertension and dyslipidemia. Comparing to general population of the same age, the severity of atherosclerosis and vascular calcification in CKD patients is intensified by the prolonged exposure to phosphate retention, the increased calcium load from calcium-based phosphate binder and high dialysate calcium and the presence of uremia and inflammation[51,52]. The prevalence of coronary artery calcification (CAC) in dialysis patients ranges between 80%-90%[53,54]. In a study that evaluated vascular calcification at the time of transplantation found the presence of CAC in 65%[55]. The pathogenesis of vascular calcification involves an active cellular process of vascular smooth muscle cell transformation into osteoblast-like cells. This programmed cellular transformation can be induced by high calcium and high phosphate environment and made worse by the reduction of calcification inhibitors that occurs in uremic environment[56]. Kidney transplantation offers a mean to improve both kidney function and abnormal mineral metabolism at the same time. Following KT recipients with good allograft function for 1-2 years after transplantation revealed a stabilization of vascular calcification in most patients[57]. However, with longer follow-up period up to 4 years, overall progression was observed[58,59]. When compared vascular calcification in patients who remained on dialysis to KT recipients, the degree of vascular calcification was more pronounced in KT recipients especially among those who had been on dialysis for longer than 2 years (Figure 2A). With increasing length of time after transplantation, worsening of vascular calcification was also observed (Figure 2B)[60]. A review of 13 studies on vascular calcification in KT recipients found that CAC continued to progress at a slow rate after transplantation. There was a strong association between baseline CAC score and CAC progression. A significant improvement in hyperparathyroidism after transplantation retarded the progression of CAC and low 25-OH-D level was an independent determinant of CAC progression[61]. Since abnormal mineral metabolism is largely restored by kidney transplantation, these data suggest that cellular changes within the vascular wall are likely to be irreversible. Moreover, the continued exposure to traditional risk factors such as diabetes, hypertension and dyslipidemia as well as corticosteroid may also encourage the progression.
Similar to CKD population, increased FGF-23 levels in KT recipients predict cardiovascular mortality, all-cause mortality and the composite outcome of allograft loss and death[62,63]. Serum phosphate greater than 3.5 mg/dL is a predictor of all-cause mortality after 4 years of follow-up independent of allograft function[64]. PTH level higher than 135 pg/mL at 10 wk after transplantation has been shown to predict the composite endpoint of cardiovascular events, graft loss and all-cause mortality[65]. PTH levels greater than 130 pg/mL at 3 mo after transplantation is an independent predictor of fracture after 5 years of follow-up[66]. In a retrospective review, high serum calcium with low serum phosphate in KT recipients was associated with a decline in graft function during the first year[67]. Low 25-OH-D level can predict all-cause mortality but not cardiovascular mortality in long-term KT recipients. This observation suggests that conditions other than cardiovascular disease, such as malignancy, may be the cause of an increased mortality[21,68]. Low 25-OH-D concentration measured 3 mo after transplantation is also an independent risk factor for interstitial fibrosis progression and is associated with lower GFR 1 year after transplantation[69]. The presence of CAC score greater than 100 and the progression of CAC in KT recipients are strongly predictive of cardiovascular events and mortality[61,70].
Kidney disease improving global outcomes (KDIGO) recommends following serum calcium and phosphate at least once a week during the first 2 mo after transplantation or until the concentrations stabilize. Thereafter, the frequency of monitoring depends on the level of allograft function. In KT recipients with eGFR ≥ 30 mL/min (stage 3T), serum calcium and phosphate should be followed every 6-12 mo and PTH should be followed yearly. Once eGFR decreases to 15-29 mL/min (stage 4T), serum calcium and phosphate should be followed every 3-6 mo and PTH every 6-12 mo. In KT recipients stage 5T, serum calcium and phosphate should be followed every 1-3 mo and PTH every 3-6 mo[71]. 25-OH-D level should also be checked in the early post-transplant period. Regarding monitoring bone loss and evaluation of fracture risk, in patients with eGFR > 30 mL/min, BMD measurement can be valuable and should be determined within the first 3 mo and then every year thereafter. If the loss of BMD is less than 5%, monitoring every 2 years is adequate[71].
Hypophosphatemia is common in the early period post-transplantation as a result of renal phosphate loss from FGF-23 excess and calcitriol deficiency[3]. Patients with mild to moderate hypophosphatemia are largely asymptomatic and phosphate replacement may do more harm than good causing a binding to calcium resulting in hypocalcemia and nephrocalcinosis[72]. Acute phosphate nephropathy has also been reported in association with oral phosphate replacement in KT recipients[73]. However, if serum phosphate drops below 1-1.5 mg/dL or patients are symptomatic, phosphate replacement may be necessary in order to alleviate the symptoms and to prevent bone demineralization. Once serum phosphate is stable, phosphate replacement should be discontinued.
Earlier studies have shown the effectiveness of oral calcitriol with or without calcium supplement in reducing PTH in KT recipients with normocalcemic hyperparathyroidism[74,75]. Calcitriol can also prevent bone loss especially during the first year after transplantation[76,77]. Similar to calcitriol, oral alfacalcidol can lower PTH and improve BMD in KT recipients[78,79]. In these studies, transient hypercalcemia occurred in a few patients but all incidences were without clinical significance. In a later randomized controlled study of paricalcitol vs no treatment for 1 year in incident KT recipients under steroid withdrawal protocol revealed the effectiveness of oral paricalcitol in lowering PTH with a 54% relative risk reduction of hyperparathyroidism. BMD increased in both groups and there was no difference in the change of BMD among the two groups. The reason for preservation of BMD in all patients was likely due to the steroid withdrawal regimen used in this study. Few patients developed hypercalcemia and/or hypercalciuria necessitating discontinuation of the drug or reduction of the dosage[80]. Another randomized controlled trial of paricalcitol vs no treatment for 6 mo in prevalent KT recipients with transplant duration 5-17 years also revealed the ability of paricalcitol in lowering PTH. In this study, vertebral BMD as well as proteinuria improved after paricalcitol therapy[81]. The above evidence indicate that oral active vitamin D are beneficial in alleviating persistent hyperparathyroidism and improving bone mass. In incident KT recipients, oral active vitamin D with or without calcium supplement for at least 6 mo to 1 year after transplantation can also prevent early post-transplant bone loss. Hypercalcemia and increased calcium load are major limiting factors for the use of active vitamin D. As for nutritional vitamin D, according to KDIGO guideline, 25-OH-D level should be measured and nutritional vitamin D should be given according to the recommendation for general population[71]. Since nutritional vitamin D supplement can provide the substrate (25-OH-D) for 1,25-OH2-D production, the decrease in PTH level was observed after cholecalciferol 25000 IU/mo or 400 IU/d supplementation. However, the benefit of nutritional vitamin D in preservation of bone mass was inconsistent[82,83].
Bisphosphonates are analogs of inorganic pyrophosphate that have the ability to suppress osteoclastic bone resorption. Bisphosphonates are commonly used in the treatment of osteoporosis in general population. Trials that evaluated the effectiveness of intravenous bisphosphonates in prevention of bone loss in KT recipients revealed the ability of intravenous ibandronate, pamidronate and zoledronic acid in preservation of BMD especially at the lumbar spine during the first year after transplantation[84-86]. Comparison between intravenous pamidronate given at baseline, months 1, 2, 3, and 6 on top of oral calcitriol and calcium carbonate to oral calcitriol and calcium alone in incident KT recipients revealed the superiority of intravenous pamidronate in preservation of vertebral BMD but all patients that received pamidronate developed adynamic bone disease at the end of the study[87]. In a randomized controlled trial comparing intravenous ibandronate every 3 mo for 12 mo to placebo on top of oral calcitriol and calcium in incident KT recipients revealed the effectiveness of ibandronate in further improving BMD at femur and ultradistal radius compared to oral calcitriol and calcium alone[88]. As for oral bisphosphonates, oral alendronate and risedronate are either superior or equally effective to oral active vitamin D in prevention of early post-transplant bone loss at the lumbar spine and the hip but failed to show benefit in the reduction fracture risk[79,89-92]. Bone biopsy study that evaluated the effect of oral risidronate for 12 mo on bone turnover in incident KT recipients revealed no evidence of adynamic bone disease[93]. The difference between this study and the intravenous pamidronate study mentioned earlier may be due to the use of a combined regimen of oral calcitriol and pamidronate or the dose and/or the route of administration of pamidronate that might have exaggerated the suppression of bone turnover. The systematic review of randomized controlled trials and the retrospective review of trials in prevention of early post-transplant bone loss revealed the superiority of a combined regimen of bisphosphonate and active vitamin D (± calcium) to active vitamin D (± calcium) alone in prevention of bone loss during the first year after transplantation but both regimens failed to show the favorable outcome in reducing fracture risk[94,95]. Another study in patients with an average transplant vintage of 2 years revealed the superiority of a combined regimen of oral risedronate on top of nutritional vitamin D (cholecalciferol) and calcium to nutritional vitamin D and calcium alone in reducing bone loss at the lumbar spine. However, the incidence of fracture was not different among the two groups[96]. In long-term KT recipients, data on the benefit of bisphosphonates appear to be inconsistent. An earlier study in KT recipients with an average transplant vintage of 9 years with osteopenia or osteoporosis at baseline revealed the same degree of effectiveness of oral alendronate and oral calcitriol in improving BMD at the lumbar spine and femur[77]. However, in a recent observational study in patients who received kidney transplantation 10 years ago, oral alendronate given for 36 mo did not improve bone mass and failed to prevent fracture[97]. According to these data, oral bisphosphonate with or without active vitamin D should be given to KT recipients with osteopenia and/or osteoporosis during the first year after kidney transplantation. Nevertheless, care should be taken in giving bisphosphonate to patients with suspected adynamic bone disease. The benefit of bisphosphonate beyond the first 1-2 years remains unclear and will require further study.
In earlier studies of steroid withdrawal, discontinuation of oral prednisolone 3 mo after transplantation resulted in a stabilization of BMD at lumbar spine and femoral neck after 3 mo of follow-up and withdrawal of prednisolone approximately one year or more after transplantation resulted in an improvement in BMD at femoral neck and total hip by 2%-3% after one year and lumbar spine by 3%-7% after 1-3 years[98-101]. A recent study in KT recipients who were managed with early corticosteroid withdrawal protocol revealed the preservation of bone mass at lumbar spine and total hip up to at least 12 mo after transplantation. The study also found the decline in cortical bone area, density and thickness and the decrease in trabecular bone density and stiffness and failure load in the distal 1/3 of radius and tibia indicating the benefit of steroid withdrawal on central skeleton but not peripheral skeleton. The loss of cortical bone was associated with the increased severity of hyperparathyroidism and the loss of trabecular bone was most severe at the lowest and highest PTH levels[102]. Unfortunately, these studies did not evaluate the impact of steroid withdrawal on fracture risk. A recent analysis of 77430 KT recipients from United States Renal Data System revealed the incidence of fracture that led to hospitalization after a median follow-up of 32 mo to be 0.0058 per patient-year in patients who did not receive steroid compared to 0.008 per patient-year in patients who received steroid. The most common fracture sites were femur (29%), ankle (15%) and spine (11%). Corticosteroid withdrawal was associated with a 31% reduction in the fracture risk[103]. According to the above data, steroid withdrawal can preserve bone mass especially in the central skeleton. A prospective study is required to confirm the benefit of steroid-sparing regimen on fracture risk.
Calcimimetic is an allosteric modulator of calcium sensing receptor that has the ability to increase the sensitivity of the receptor to calcium and suppress PTH secretion. Cinacalcet, the only drug in this class, is used as an add-on therapy to active vitamin D in the treatment of secondary hyperparathyroidism in CKD. Discontinuation of cinacalcet at the time of transplantation can cause rebound hypercalcemia and hyperparathyroidism resulting in an increase in the incidence of post-transplant nephrocalcinosis and parathyroidectomy and, therefore, stopping the drug immediately after transplantation is not recommended[20,104]. Since a decade ago, cinacalcet has been utilized as an alternative therapy to parathyroidectomy in KT recipients with hypercalcemia due to persistent hyperparathyroidism[105,106]. After initiation of cinacalcet, serum calcium decreased, serum phosphate increased and hyperparathyroidism improved without a significant change in serum creatinine. The increase in serum phosphate helps keeping serum phosphate within the normal range. A systematic review and meta-analysis of 411 KT recipients confirms the effectiveness of cinacalcet in controlling hypercalcemia and hyperparathyroidism after kidney transplantation[107]. Despite the improvement in hyperparathyroidism, a two-year therapy with cinacalcet in prevalent KT recipients did not result in an improvement in BMD[108,109]. Few reports have described the development of hypercalciuria and nephrolithiasis in the kidney allograft after cinacalcet therapy. Nephrolithiasis resolved after discontinuing cinacalcet and parathyroidectomy suggesting the role of hyperparathyroidism in addition to cinacalcet alone in kidney stone formation[110,111]. Long-term data on cinacalcet therapy up to 6 years revealed safety and effectiveness of the drug in the treatment of hypercalcemia and hyperparathyroidism. Discontinuation of cinacalcet after 3.5 years of continuous therapy resulted in an increase in serum calcium with one-third of the patients requiring re-initiation of the drug due to hypercalcemia[112]. Overall cinacalcet is a safe and effective therapy for hypercalcemia and persistent hyperparathyroidism after kidney transplantation and the effectiveness of cinacalcet is maintained for several years. There is no cut point as to how long the therapy should be continued since the severity and duration of hyperparathyroidism varies from patient to patient and, therefore, the time required for the shrinkage of enlarged hyperplastic parathyroid glands differs among patients.
In the past, surgical treatment for persistent hyperparathyroidism after kidney transplantation has been either total parathyroidectomy with autotransplantation or subtotal (3.5 glands) to near total parathyroidectomy. Limited glandular resection is advocated in patients presenting with only one or two macroscopically enlarged glands. Choices of preoperative localization study include 99mTc-sestimibi scintigraphy, ultrasound, computed tomography and magnetic resonance imaging. In a single-center study, both total parathyroidectomy with autotransplantation and subtotal parathyroidectomy were equally effective in alleviating hyperparathyroidism and hypercalcemia but patients who underwent total parathyroidectomy showed a tendency toward lower PTH levels with an increased risk of hypoparathyroidism[113]. Subtotal to near total parathyroidectomy is now a standard surgery for persistent hyperparathyroidism after kidney transplantation. A recent retrospective review revealed near total parathyroidectomy resulting in a resolution of 96.9% of patients’ hypercalcemia with 78.4% of the patients had PTH level below 250 pg/mL after a median follow-up of 3 years[114]. After parathyroidectomy, deterioration of allograft function is common with a 5%-30% drop in eGFR. The severity of baseline hyperparathyroidism seems to predict the decline in eGFR after surgery. Recovery of allograft function may be expected after 12 mo[115]. However, long-term follow-up data comparing eGFR in patients who underwent parathyroidectomy during the first year after transplantation to those who had surgery prior to transplantation revealed a significantly lower eGFR after 5 years in patients who had parathyroidectomy after transplantation[116]. Due to this evidence, several centers consider performing parathyroidectomy during the waiting period in patients with severe hyperparathyroidism associated with hypercalcemia. However, the cut-off values for PTH and serum calcium during the waiting period are not clearly defined. In one retrospective review, patients who required parathyroidectomy after transplantation had an average PTH level of 723 pg/mL (range 557-919) 1 year prior to transplantation whereas those who did not require surgery had an average PTH level of 212 pg/mL (range 160-439)[18]. The data on parathyroidectomy in end-stage renal disease patients on the waiting list indicated that total parathyroidectomy with autotransplantation could cause permanent hypocalcemia in 50%-83% after transplantation whereas less-than-total parathyroidectomy resulted in normocalcemia in all patients[117]. After parathyroidectomy, it is better to wait until serum calcium and phosphate are stable and hungry bone syndrome subsides before proceeding to kidney transplantation in order to avoid intractable hypocalcemia postoperatively. In those who did not undergo surgery prior to transplantation, there is currently no consensus as to when parathyroidectomy should be performed. It is recommended that, during the first year, physicians should try to manage hypercalcemia and hyperparathyroidism with available medications in order to allow the time for the shrinkage of hyperplastic parathyroid glands to occur[118,119]. If patients continue to have hypercalcemia with elevated PTH levels despite the use of active vitamin D and/or calcimimetic after 1 year, display a continuous decline in BMD or develop a fracture or nephrolithiasis in the kidney allograft, in these cases, parathyroidectomy should be considered. If hypoparathyroidism develops after kidney transplantation, methods that have been used to correct hypocalcemia in addition to calcium and calcitriol include daily teriparatide injection, the use of parathyroid tissue that has been cryopreserved at the time of surgery for a metachronous autotransplantation or parathyroid allotransplantation from a well-matched living or cadaveric donor[120-122].
Despite the improvement in mineral metabolites and mineral regulating hormones after kidney transplantation, abnormal bone and mineral metabolism continues to present in most patients. High dose corticosteroid and persistent hyperparathyroidism are the most important factors influencing abnormal bone and mineral metabolism after kidney transplantation. The use of active vitamin D with or without bisphosphonate is effective in preventing early post-transplant bone loss. Steroid withdrawal regimen is also beneficial in preservation of bone mass in long-term. Calcimimetic is an alternative therapy to parathyroidectomy in KT recipients with persistent hyperparathyroidism. If parathyroidectomy is required, subtotal to near total parathyroidectomy appears to result in a more favorable long-term outcome compared to total parathyroidectomy with autotransplantation. Performing parathyroidectomy during the waiting period prior to transplantation is also preferred in patients with severe hyperparathyroidism associated with hypercalcemia.
P- Reviewer: Ohashi N, Stavroulopoulos A
S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SM, Jüppner H, Wolf M. Post-transplant hypophosphatemia: Tertiary ‘Hyper-Phosphatoninism’? Kidney Int. 2006;70:1486-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Pande S, Ritter CS, Rothstein M, Wiesen K, Vassiliadis J, Kumar R, Schiavi SC, Slatapolsky E, Brown AJ. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Physiol. 2006;104:p23-p32. [PubMed] |
| 3. | Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y. Tertiary ‘hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant. 2007;7:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Barros X, Torregrosa JV, Martínez de Osaba MJ, Casals G, Paschoalin R, Durán CE, Campistol JM. Earlier decrease of FGF-23 and less hypophosphatemia in preemptive kidney transplant recipients. Transplantation. 2012;94:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Evenepoel P, Meijers BK, de Jonge H, Naesens M, Bammens B, Claes K, Kuypers D, Vanrenterghem Y. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol. 2008;3:1829-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Kawarazaki H, Shibagaki Y, Fukumoto S, Kido R, Nakajima I, Fuchinoue S, Fujita T, Fukagawa M, Teraoka S. The relative role of fibroblast growth factor 23 and parathyroid hormone in predicting future hypophosphatemia and hypercalcemia after living donor kidney transplantation: a 1-year prospective observational study. Nephrol Dial Transplant. 2011;26:2691-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Bleskestad IH, Thorsen IS, Jonsson G, Skadberg Ø, Bergrem H, Gøransson LG. Soluble Klotho and intact fibroblast growth factor 23 in long-term kidney transplant patients. Eur J Endocrinol. 2015;172:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Sirilak S, Chatsrisak K, Ingsathit A, Kantachuvesiri S, Sumethkul V, Stitchantrakul W, Radinahamed P, Disthabanchong S. Renal phosphate loss in long-term kidney transplantation. Clin J Am Soc Nephrol. 2012;7:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Tomida K, Hamano T, Ichimaru N, Fujii N, Matsui I, Nonomura N, Tsubakihara Y, Rakugi H, Takahara S, Isaka Y. Dialysis vintage and parathyroid hormone level, not fibroblast growth factor-23, determines chronic-phase phosphate wasting after renal transplantation. Bone. 2012;51:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 631] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 11. | Messa P, Sindici C, Cannella G, Miotti V, Risaliti A, Gropuzzo M, Di Loreto PL, Bresadola F, Mioni G. Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int. 1998;54:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Muirhead N, Zaltman JS, Gill JS, Churchill DN, Poulin-Costello M, Mann V, Cole EH. Hypercalcemia in renal transplant patients: prevalence and management in Canadian transplant practice. Clin Transplant. 2014;28:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kawarazaki H, Shibagaki Y, Fukumoto S, Kido R, Ando K, Nakajima I, Fuchinoue S, Fujita T, Fukagawa M, Teraoka S. Natural history of mineral and bone disorders after living-donor kidney transplantation: a one-year prospective observational study. Ther Apher Dial. 2011;15:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Torres A, Rodríguez AP, Concepción MT, García S, Rufino M, Martín B, Pérez L, Machado M, de Bonis E, Losada M. Parathyroid function in long-term renal transplant patients: importance of pre-transplant PTH concentrations. Nephrol Dial Transplant. 1998;13 Suppl 3:94-97. [PubMed] |
| 15. | Nakamura M, Tanaka K, Marui Y, Tomikawa S. Clinicopathological analysis of persistent hypercalcemia and hyperparathyroidism after kidney transplantation in long-term dialysis patients. Ther Apher Dial. 2013;17:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Torregrosa JV, Fuster D, Duran CE, Oppenheimer F, Muxí Á, Rubello D, Pons F, Campistol JM. Set point of calcium in severe secondary hyperparathyroidism is altered and does not change after successful kidney transplantation. Endocrine. 2015;48:709-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Dewberry LC, Tata S, Graves S, Weber CJ, Sharma J. Predictors of tertiary hyperparathyroidism: Who will benefit from parathyroidectomy? Surgery. 2014;156:1631-1636; discussion 1636-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Taniguchi M, Tokumoto M, Matsuo D, Motoyama K, Sugitani A, Kuroki S, Yotsueda H, Tsuruya K, Hirakata H, Iida M. Persistent hyperparathyroidism in renal allograft recipients: vitamin D receptor, calcium-sensing receptor, and apoptosis. Kidney Int. 2006;70:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Evenepoel P, Sprangers B, Lerut E, Bammens B, Claes K, Kuypers D, Meijers B, Vanrenterghem Y. Mineral metabolism in renal transplant recipients discontinuing cinacalcet at the time of transplantation: a prospective observational study. Clin Transplant. 2012;26:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Keyzer CA, Riphagen IJ, Joosten MM, Navis G, Muller Kobold AC, Kema IP, Bakker SJ, de Borst MH. Associations of 25(OH) and 1,25(OH)2 vitamin D with long-term outcomes in stable renal transplant recipients. J Clin Endocrinol Metab. 2015;100:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Ewers B, Gasbjerg A, Moelgaard C, Frederiksen AM, Marckmann P. Vitamin D status in kidney transplant patients: need for intensified routine supplementation. Am J Clin Nutr. 2008;87:431-437. [PubMed] |
| 23. | Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J. 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab. 2006;91:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD. Vitamin D status in renal transplant recipients. Am J Transplant. 2007;7:2546-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Penny H, Frame S, Dickinson F, Garrett G, Young AR, Sarkany R, Chitalia N, Hampson G, Goldsmith D. Determinants of vitamin D status in long-term renal transplant patients. Clin Transplant. 2012;26:E617-E623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Eyal O, Aharon M, Safadi R, Elhalel MD. Serum vitamin D levels in kidney transplant recipients: the importance of an immunosuppression regimen and sun exposure. Isr Med Assoc J. 2013;15:628-633. [PubMed] |
| 27. | Beique LC, Kline GA, Dalton B, Duggan K, Yilmaz S. Predicting deficiency of vitamin D in renal transplant recipients in northern climates. Transplantation. 2013;95:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kulshrestha S, Ojo AO, Luan FL. Metabolic syndrome, vitamin D deficiency and hypoadiponectinemia among nondiabetic patients early after kidney transplantation. Am J Nephrol. 2013;37:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Falkiewicz K, Boratynska M, Speichert-Bidzińska B, Magott-Procelewska M, Biecek P, Patrzalek D, Klinger M. 1,25-dihydroxyvitamin D deficiency predicts poorer outcome after renal transplantation. Transplant Proc. 2009;41:3002-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Adorini L, Amuchastegui S, Daniel KC. Prevention of chronic allograft rejection by Vitamin D receptor agonists. Immunol Lett. 2005;100:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Young MR, Day TA. Immune regulatory activity of vitamin d3 in head and neck cancer. Cancers (Basel). 2013;5:1072-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | McGregor R, Li G, Penny H, Lombardi G, Afzali B, Goldsmith DJ. Vitamin D in renal transplantation - from biological mechanisms to clinical benefits. Am J Transplant. 2014;14:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Nobata H, Tominaga Y, Imai H, Uchida K. Hypocalcemia immediately after renal transplantation. Clin Transplant. 2013;27:E644-E648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Chatzikyrkou C, Clajus C, Haubitz M, Hafer C. Hypercalcemia and pneumocystis Pneumonia after kidney transplantation: report of an exceptional case and literature review. Transpl Infect Dis. 2011;13:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Ramalho J, Bacelar Marques ID, Aguirre AR, Pierrotti LC, de Paula FJ, Nahas WC, David-Neto E. Pneumocystis jirovecii pneumonia with an atypical granulomatous response after kidney transplantation. Transpl Infect Dis. 2014;16:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Bency R, Roger SD, Elder GJ. Hypercalcaemia as a prodromal feature of indolent Pneumocystis jivorecii after renal transplantation. Nephrol Dial Transplant. 2011;26:1740-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Cohen A, Sambrook P, Shane E. Management of bone loss after organ transplantation. J Bone Miner Res. 2004;19:1919-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 442] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Lim DS, Kee TY, Fook-Chong S, Zhang RF, Chandran M. Prevalence and patterns of bone loss in the first year after renal transplant in South East Asian patients. Transplantation. 2011;92:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Kalantar-Zadeh K, Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S. Management of mineral and bone disorder after kidney transplantation. Curr Opin Nephrol Hypertens. 2012;21:389-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol. 2000;11:1093-1099. [PubMed] |
| 42. | Rojas E, Carlini RG, Clesca P, Arminio A, Suniaga O, De Elguezabal K, Weisinger JR, Hruska KA, Bellorin-Font E. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 2003;63:1915-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Casez JP, Lippuner K, Horber FF, Montandon A, Jaeger P. Changes in bone mineral density over 18 months following kidney transplantation: the respective roles of prednisone and parathyroid hormone. Nephrol Dial Transplant. 2002;17:1318-1326. [PubMed] |
| 44. | Canalis E. Mechanisms of glucocorticoid-induced osteoporosis. Curr Opin Rheumatol. 2003;15:454-457. [PubMed] |
| 45. | Lehmann G, Ott U, Stein G, Steiner T, Wolf G. Renal osteodystrophy after successful renal transplantation: a histomorphometric analysis in 57 patients. Transplant Proc. 2007;39:3153-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Dolgos S, Hartmann A, Bønsnes S, Ueland T, Isaksen GA, Godang K, Pfeffer P, Bollerslev J. Determinants of bone mass in end-stage renal failure patients at the time of kidney transplantation. Clin Transplant. 2008;22:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Huang WH, Yu MC, Huang JY, Lai PC. Impact of hepatitis C virus infection on bone mineral density in renal transplant recipients. PLoS One. 2013;8:e63263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288:3014-3018. [PubMed] |
| 49. | Vautour LM, Melton LJ, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: a population-based study. Osteoporos Int. 2004;15:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Evenepoel P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin Nephrol. 2013;33:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112-S119. [PubMed] |
| 52. | London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731-1740. [PubMed] |
| 53. | Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 1939] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 54. | Disthabanchong S, Jongjirasiri S, Adirekkiat S, Sumethkul V, Ingsathit A, Domrongkitchaiporn S, Phakdeekitcharoen B, Kantachuvesiri S, Kitiyakara C. Low hip bone mineral density predicts mortality in maintenance hemodialysis patients: a five-year follow-up study. Blood Purif. 2014;37:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Rosas SE, Mensah K, Weinstein RB, Bellamy SL, Rader DJ. Coronary artery calcification in renal transplant recipients. Am J Transplant. 2005;5:1942-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Disthabanchong S. Vascular calcification in chronic kidney disease: Pathogenesis and clinical implication. World J Nephrol. 2012;1:43-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Bargnoux AS, Dupuy AM, Garrigue V, Jaussent I, Gahide G, Badiou S, Szwarc I, Deleuze S, Vernhet H, Cristol JP. Evolution of coronary artery calcifications following kidney transplantation: relationship with osteoprotegerin levels. Am J Transplant. 2009;9:2571-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Maréchal C, Coche E, Goffin E, Dragean A, Schlieper G, Nguyen P, Floege J, Kanaan N, Devuyst O, Jadoul M. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. 2012;59:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Seyahi N, Cebi D, Altiparmak MR, Akman C, Ataman R, Pekmezci S, Serdengecti K. Progression of coronary artery calcification in renal transplant recipients. Nephrol Dial Transplant. 2012;27:2101-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Vipattawat K, Kitiyakara C, Phakdeekitcharoen B, Kantachuvesiri S, Sumethkul V, Jirasiritham S, Stitchantrakul W, Disthabanchong S. Vascular calcification in long-term kidney transplantation. Nephrology (Carlton). 2014;19:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Cianciolo G, Capelli I, Angelini ML, Valentini C, Baraldi O, Scolari MP, Stefoni S. Importance of vascular calcification in kidney transplant recipients. Am J Nephrol. 2014;39:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 63. | Baia LC, Humalda JK, Vervloet MG, Navis G, Bakker SJ, de Borst MH. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol. 2013;8:1968-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 64. | Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP. Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation. 2009;87:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Bleskestad IH, Bergrem H, Leivestad T, Hartmann A, Gøransson LG. Parathyroid hormone and clinical outcome in kidney transplant patients with optimal transplant function. Clin Transplant. 2014;28:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | Perrin P, Caillard S, Javier RM, Braun L, Heibel F, Borni-Duval C, Muller C, Olagne J, Moulin B. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant. 2013;13:2653-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Hiemstra TF, Brown AJ, Chaudhry AN, Walsh M. Association of calcium, phosphate and parathyroid hormone with renal allograft function: a retrospective cohort study. Am J Nephrol. 2013;37:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Marcén R, Jimenez S, Fernández-Rodriguez A, Galeano C, Villafruela JJ, Gomis A, Teruel JL, Quereda C. Are low levels of 25-hydroxyvitamin D a risk factor for cardiovascular diseases or malignancies in renal transplantation? Nephrol Dial Transplant. 2012;27 Suppl 4:iv47-iv52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Bienaimé F, Girard D, Anglicheau D, Canaud G, Souberbielle JC, Kreis H, Noël LH, Friedlander G, Elie C, Legendre C. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol. 2013;24:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Roe P, Wolfe M, Joffe M, Rosas SE. Inflammation, coronary artery calcification and cardiovascular events in incident renal transplant recipients. Atherosclerosis. 2010;212:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;S1-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 1067] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 72. | Evenepoel P, Lerut E, Naesens M, Bammens B, Claes K, Kuypers D, Vermeersch P, Meijers B, Van Damme B, Vanrenterghem Y. Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am J Transplant. 2009;9:2470-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Riella LV, Rennke HG, Grafals M, Chandraker A. Hypophosphatemia in kidney transplant recipients: report of acute phosphate nephropathy as a complication of therapy. Am J Kidney Dis. 2011;57:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Lobo PI, Cortez MS, Stevenson W, Pruett TL. Normocalcemic hyperparathyroidism associated with relatively low 1: 25 vitamin D levels post-renal transplant can be successfully treated with oral calcitriol. Clin Transplant. 1995;9:277-281. [PubMed] |
| 75. | Steiner RW, Ziegler M, Halasz NA, Catherwood BD, Manolagas S, Deftos LJ. Effect of daily oral vitamin D and calcium therapy, hypophosphatemia, and endogenous 1-25 dihydroxycholecalciferol on parathyroid hormone and phosphate wasting in renal transplant recipients. Transplantation. 1993;56:843-846. [PubMed] |
| 76. | Josephson MA, Schumm LP, Chiu MY, Marshall C, Thistlethwaite JR, Sprague SM. Calcium and calcitriol prophylaxis attenuates posttransplant bone loss. Transplantation. 2004;78:1233-1236. [PubMed] |
| 77. | Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: a randomized prospective trial of calcitriol versus alendronate. Transplantation. 2003;76:1498-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | El-Husseini AA, El-Agroudy AE, El-Sayed MF, Sobh MA, Ghoneim MA. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatr Transplant. 2004;8:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | El-Agroudy AE, El-Husseini AA, El-Sayed M, Mohsen T, Ghoneim MA. A prospective randomized study for prevention of postrenal transplantation bone loss. Kidney Int. 2005;67:2039-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Amer H, Griffin MD, Stegall MD, Cosio FG, Park WD, Kremers WK, Heilman RL, Mazur MJ, Hamawi K, Larson TS. Oral paricalcitol reduces the prevalence of posttransplant hyperparathyroidism: results of an open label randomized trial. Am J Transplant. 2013;13:1576-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Trillini M, Cortinovis M, Ruggenenti P, Reyes Loaeza J, Courville K, Ferrer-Siles C, Prandini S, Gaspari F, Cannata A, Villa A. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J Am Soc Nephrol. 2015;26:1205-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Wissing KM, Broeders N, Moreno-Reyes R, Gervy C, Stallenberg B, Abramowicz D. A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation. 2005;79:108-115. [PubMed] |
| 83. | Sahin G, Yasar NS, Sirmagul B, Bal C, Yalcin AU. The effect of low-dose cholecalciferol and calcium treatment on posttransplant bone loss in renal transplant patients: a prospective study. Ren Fail. 2008;30:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, Strey C, Kirste G, Olschewski M, Reichelt A. Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol. 2001;12:1530-1537. [PubMed] |
| 85. | Fan SL, Almond MK, Ball E, Evans K, Cunningham J. Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int. 2000;57:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 86. | Haas M, Leko-Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Mitterbauer C, Steininger R, Grampp S, Klaushofer K, Delling G. Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney Int. 2003;63:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, Tellis V, Greenstein S, Schechner R, Figueroa K. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;14:2669-2676. [PubMed] |
| 88. | Smerud KT, Dolgos S, Olsen IC, Åsberg A, Sagedal S, Reisæter AV, Midtvedt K, Pfeffer P, Ueland T, Godang K. A 1-year randomized, double-blind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant. 2012;12:3316-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Nowacka-Cieciura E, Cieciura T, Baczkowska T, Kozińska-Przybył O, Tronina O, Chudziński W, Pacholczyk M, Durlik M. Bisphosphonates are effective prophylactic of early bone loss after renal transplantation. Transplant Proc. 2006;38:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Huang WH, Lee SY, Weng CH, Lai PC. Use of alendronate sodium (Fosamax) to ameliorate osteoporosis in renal transplant patients: a case-control study. PLoS One. 2012;7:e48481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Torregrosa JV, Fuster D, Gentil MA, Marcen R, Guirado L, Zarraga S, Bravo J, Burgos D, Monegal A, Muxí A. Open-label trial: effect of weekly risedronate immediately after transplantation in kidney recipients. Transplantation. 2010;89:1476-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Mainra R, Elder GJ. Individualized therapy to prevent bone mineral density loss after kidney and kidney-pancreas transplantation. Clin J Am Soc Nephrol. 2010;5:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Coco M, Pullman J, Cohen HW, Lee S, Shapiro C, Solorzano C, Greenstein S, Glicklich D. Effect of risedronate on bone in renal transplant recipients. J Am Soc Nephrol. 2012;23:1426-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Palmer SC, Strippoli GF, McGregor DO. Interventions for preventing bone disease in kidney transplant recipients: a systematic review of randomized controlled trials. Am J Kidney Dis. 2005;45:638-649. [PubMed] |
| 95. | Jeon HJ, Han M, Jeong JC, Kim YJ, Kwon HY, Koo TY, Ahn C, Yang J. Impact of vitamin D, bisphosphonate, and combination therapy on bone mineral density in kidney transplant patients. Transplant Proc. 2013;45:2963-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Torregrosa JV, Fuster D, Pedroso S, Diekmann F, Campistol JM, Rubí S, Oppenheimer F. Weekly risedronate in kidney transplant patients with osteopenia. Transpl Int. 2007;20:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Yamamoto S, Suzuki A, Sasaki H, Sekiguchi-Ueda S, Asano S, Shibata M, Hayakawa N, Hashimoto S, Hoshinaga K, Itoh M. Oral alendronate can suppress bone turnover but not fracture in kidney transplantation recipients with hyperparathyroidism and chronic kidney disease. J Bone Miner Metab. 2013;31:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | van den Ham EC, Kooman JP, Christiaans ML, van Hooff JP. The influence of early steroid withdrawal on body composition and bone mineral density in renal transplantation patients. Transpl Int. 2003;16:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 99. | Pelletier SJ, Vanderwall K, Debroy MA, Englesbe MJ, Sung RS, Magee JC, Fontana RJ, Punch JD. Preliminary analysis of early outcomes of a prospective, randomized trial of complete steroid avoidance in liver transplantation. Transplant Proc. 2005;37:1214-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Farmer CK, Hampson G, Abbs IC, Hilton RM, Koffman CG, Fogelman I, Sacks SH. Late low-dose steroid withdrawal in renal transplant recipients increases bone formation and bone mineral density. Am J Transplant. 2006;6:2929-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Ing SW, Sinnott LT, Donepudi S, Davies EA, Pelletier RP, Lane NE. Change in bone mineral density at one year following glucocorticoid withdrawal in kidney transplant recipients. Clin Transplant. 2011;25:E113-E123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Iyer SP, Nikkel LE, Nishiyama KK, Dworakowski E, Cremers S, Zhang C, McMahon DJ, Boutroy S, Liu XS, Ratner LE. Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol. 2014;25:1331-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Nikkel LE, Mohan S, Zhang A, McMahon DJ, Boutroy S, Dube G, Tanriover B, Cohen D, Ratner L, Hollenbeak CS. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant. 2012;12:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Barros X, Fuster D, Paschoalin R, Oppenheimer F, Rubello D, Perlaza P, Pons F, Torregrosa JV. Changes in bone mineral metabolism parameters, including FGF23, after discontinuing cinacalcet at kidney transplantation. Endocrine. 2015;49:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Szwarc I, Argilés A, Garrigue V, Delmas S, Chong G, Deleuze S, Mourad G. Cinacalcet chloride is efficient and safe in renal transplant recipients with posttransplant hyperparathyroidism. Transplantation. 2006;82:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 106. | Serra AL, Savoca R, Huber AR, Hepp U, Delsignore A, Hersberger M, Wüthrich RP. Effective control of persistent hyperparathyroidism with cinacalcet in renal allograft recipients. Nephrol Dial Transplant. 2007;22:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Cohen JB, Gordon CE, Balk EM, Francis JM. Cinacalcet for the treatment of hyperparathyroidism in kidney transplant recipients: a systematic review and meta-analysis. Transplantation. 2012;94:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Evenepoel P, Cooper K, Holdaas H, Messa P, Mourad G, Olgaard K, Rutkowski B, Schaefer H, Deng H, Torregrosa JV. A randomized study evaluating cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. Am J Transplant. 2014;14:2545-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Torregrosa JV, Morales E, Díaz JM, Crespo J, Bravo J, Gómez G, Gentil MÁ, Rodríguez Benot A, García MR, Jiménez VL. Cinacalcet for hypercalcaemic secondary hyperparathyroidism after renal transplantation: a multicentre, retrospective, 3-year study. Nephrology (Carlton). 2014;19:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Zitt E, Woess E, Mayer G, Lhotta K. Effect of cinacalcet on renal electrolyte handling and systemic arterial blood pressure in kidney transplant patients with persistent hyperparathyroidism. Transplantation. 2011;92:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Seager CM, Srinivas TR, Flechner SM. Development of nephrolithiasis in a renal transplant patient during treatment with Cinacalcet. Ann Transplant. 2013;18:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Thiem U, Gessl A, Borchhardt K. Long-term clinical practice experience with cinacalcet for treatment of hypercalcemic hyperparathyroidism after kidney transplantation. Biomed Res Int. 2015;2015:292654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Park JH, Kang SW, Jeong JJ, Nam KH, Chang HS, Chung WY, Park CS. Surgical treatment of tertiary hyperparathyroidism after renal transplantation: a 31-year experience in a single institution. Endocr J. 2011;58:827-833. [PubMed] |
| 114. | Dewberry LK, Weber C, Sharma J. Near total parathyroidectomy is effective therapy for tertiary hyperparathyroidism. Am Surg. 2014;80:646-651. [PubMed] |
| 115. | Parikh S, Nagaraja H, Agarwal A, Samavedi S, Von Visger J, Nori U, Andreoni K, Pesavento T, Singh N. Impact of post-kidney transplant parathyroidectomy on allograft function. Clin Transplant. 2013;27:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Jeon HJ, Kim YJ, Kwon HY, Koo TY, Baek SH, Kim HJ, Huh WS, Huh KH, Kim MS, Kim YS. Impact of parathyroidectomy on allograft outcomes in kidney transplantation. Transpl Int. 2012;25:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Jäger MD, Emmanouilidis N, Jackobs S, Kespohl H, Hett J, Musatkin D, Tränkenschuh W, Schrem H, Klempnauer J, Scheumann GF. Presence of small parathyroid glands in renal transplant patients supports less-than-total parathyroidectomy to treat hypercalcemic hyperparathyroidism. Surgery. 2014;155:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 118. | Torregrosa JV, Barros X. Management of hypercalcemia after renal transplantation. Nefrologia. 2013;33:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 119. | Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis. 2013;61:310-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 120. | Nogueira EL, Costa AC, Santana A, Guerra JO, Silva S, Mil-Homens C, Costa AG. Teriparatide efficacy in the treatment of severe hypocalcemia after kidney transplantation in parathyroidectomized patients: a series of five case reports. Transplantation. 2011;92:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Schneider R, Ramaswamy A, Slater EP, Bartsch DK, Schlosser K. Cryopreservation of parathyroid tissue after parathyroid surgery for renal hyperparathyroidism: does it really make sense? World J Surg. 2012;36:2598-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 122. | Giulianotti PC, D’Amico G, Tzvetanov I, Benedetti E. Living donor parathyroid allotransplantation with robotic transaxillary procurement in a kidney transplant recipient. Transpl Int. 2014;27:e43-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |