Published online Dec 24, 2013. doi: 10.5500/wjt.v3.i4.48
Revised: August 16, 2013
Accepted: August 28, 2013
Published online: December 24, 2013
Processing time: 158 Days and 13 Hours
Islet transplantation (IT) is today a well-established treatment modality for selected patients with type 1 diabetes mellitus (T1DM). After the success of the University of Alberta group with a modified approach to the immune protection of islets, the international experience grew along with the numbers of transplants in highly specialized centers. Yet, long-term analysis of those initial results from the Edmonton group indicated that insulin-independence was not durable and most patients return to modest amounts of insulin around the fifth year, without recurrent hypoglycemia events. Many phenomena have been identified as limiting factor for the islet engraftment and survival, and today all efforts are aimed to improve the quality of islets and their engrafting process, as well as more optimized immunosuppression to facilitate tolerance and ultimately, better long term survival. This brief overview presents recent progress in IT. A concise historical perspective is provided, along with the latest efforts to improve islet engraftment, immune protection and ultimately, prolonged graft survival. It is apparent that as the community continues to work together further optimizing IT, it is hopeful a cure for T1DM will soon be achievable.
Core tip: Since the initial inception of the “Edmonton protocol”, phenomenal progress has transpired in the last decade. These milestones were namely due to the implementation of numerous pre-clinical and clinical investigations, testing innovative agents allowing potent immunotolerance with minimal complications as well as alternative transplant sites to overcome limitations inherent to the current intraportal access. As a result nearly 80% of full or partial graft function, out of more than 300 transplants performed to date. As the field of continues to work and progress together, it is foreseeable that a cure for type 1 diabetes mellitus is obtainable in the near future.
- Citation: Pepper AR, Gala-Lopez B, Ziff O, Shapiro AJ. Current status of clinical islet transplantation. World J Transplant 2013; 3(4): 48-53
- URL: https://www.wjgnet.com/2220-3230/full/v3/i4/48.htm
- DOI: https://dx.doi.org/10.5500/wjt.v3.i4.48
Islet transplantation (IT) is today an accepted modality to treat selected diabetic patients with frequent hypoglycemics and severe glycemic lability[1,2]. The “Edmonton Protocol” became a milestone by reporting sustained C-peptide production and high rates of insulin-independence after transplant in type 1 diabetes mellitus (T1DM)[3]. This reality became possible with the use of newer, more potent immunosuppressant (IS) agents, the avoidance of corticosteroids, and high-quality islet preparations, although typically two islet infusions were required to attain insulin independence.
Long-term analysis of these initial results from the Edmonton group indicated that insulin-independence was not durable and most patients return to moderate amounts of insulin approximately 5 years post-infusion, in the absences of recurrent hypoglycemia events[4,5].
Causes for this chronic graft function remain unclear, but are likely associated with immune rejection, recurrence of autoimmunity or chronic exposure to diabetogenic IS agents[5,6].
This brief overview presents recent progress in IT. A succinct historical viewpoint is provided, along with the resent efforts to improve islet engraftment, immune protection and ultimately, prolonged graft survival.
The history of IT is filled with numerous sacrifices and hardly fought successes. Early rudimentary experiments in the 19th Century lead to the concept of isolation and purification[5]. In 1966, the University of Minnesota group performed the first clinical attempt to cure T1DM by whole pancreas transplant[7,8]. It allowed technical improvements, but more importantly, refinements in IS while introducing cyclosporine continued with the use of multiple and more potent drug schemes.
Clinical investigators at Washington University demonstrated the possibility of reversing diabetes with temporary insulin independence after transplantation of human islets. It was a transient success because IS was still insufficient[9]. A year later, the first successful series of human islet allografts was reported by the University of Pittsburgh, achieving prolonged insulin-independence with a more optimized IS based on the recently introduced agent FK-506 and no steroids[9].
Another important milestone was the report from the University of Alberta group showing successful long-term results on selected patients, with the use of a novel IS scheme. Grafts were non-human leukocyte antigen (HLA) matched, patients were not sensitized (negative panel reactive antibody pre-transplant), islets were ABO compatible, and sequential transplants were used to deliver an adequate islet infusion mass by a percutaneous portal venous access route. Immunosuppression was tailored to avoid steroids and minimize calcineurin inhibitors to prevent diabetogenicity, with the combination of sirolimus, low-dose tacrolimus (TAC), and the daclizumab induction[10].
New programs proliferated worldwide based on the lessons learned from the “Edmonton Protocol” and the number of transplant significantly increased over the coming years. However, insulin independence was not durable long-term and most patient returned to modest amounts of insulin without risk of recurrent hypoglycemia by the third to fifth year. Additionally, approximately 25% required additional late islet infusions during the second or third year post-transplant[2].
New efforts are now aimed to improve the quality of islets, enhanced their engraftment conditions and prolonged their function. Moreover, new transplant sites are also consider overcoming the limitations of the traditional intraportal site and providing a suitable framework for future strategies, such as the use of insulin-producing stem cells as surrogate for the precious and increasingly scarce human islets.
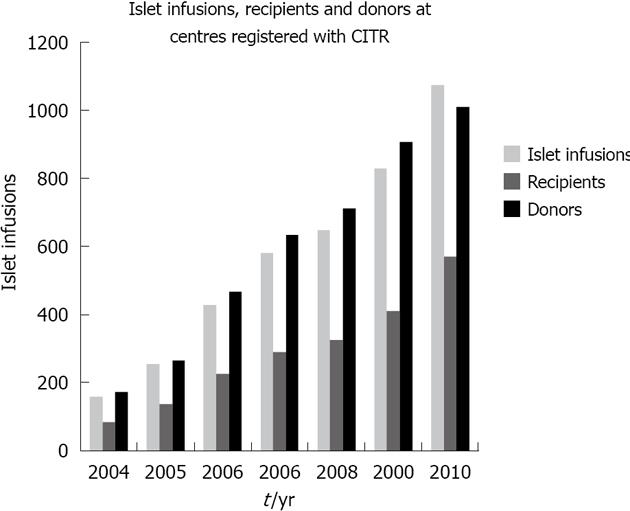
Despite significant improvements in the care of T1DM patients, a subgroup remains in significant disadvantage due to refractory hypoglycemia. The option of IT offers the possibility of improved glycemic control[2]. The recent years have witnessed substantial progress in the number and results of IT (Figure 1).
Before the year 2000, few centers performing IT achieved high rates of sustainable insulin independence after this procedure[2]. In 2000, Shapiro et al[3] reported their initial findings in seven consecutive subjects treated with glucocorticoid-free immunosuppressive therapy combined with infusion of an adequate mass of freshly prepared, bringing a new perspective on the immunoprotection provided for these patients[3]. The success achieved with this new scheme prompted interest and enthusiasm among various programs and launched a major international trial with key results for our current concepts on immunosuppression.
Today, the Clinical Islet Transplant Program at the University of Alberta remains as one of the most important and active transplant center in the world after becoming a beacon with one of the most integral and successful approaches to IT with sustained and reproducible long-term results. The task remains improving the viability of islet preparations and also in determining the most optimal IS agents to improve the initial results published with the “Edmonton Protocol”, but also to achieve single donor insulin-independence, safety and tolerability.
A recent study published a cross-sectional analysis of the current Edmonton results. It showed 79% of full or partial graft function out of more than 300 transplants performed. The median duration of insulin independence was 34.6 and 11.0 mo for subjects with full or partial graft function, whereas the duration of C-peptide was 53.3 and 70.4 mo for those same patients[11].
Phenomenal progress has occurred in the last years due to the implementation of numerous findings from pre-clinical and clinical investigations testing different agents to allow better immunotolerance with lesser complications, novel devices to provide islets with a safer environment, as well as new transplant sites to overcome limitations inherent to the current intraportal access (Table 1).
| Trial ID | Description | Institution |
| NCT01653899 | Caspase Inhibition in Islet Transplantation | University of Alberta |
| NCT00468117 | Efficacy of Islet After Kidney Transplantation | National Institute of Allergy and Infectious Diseases |
| NCT01705899 | Islet Allotransplantation in Type 1 Diabetes | Ohio State University |
| NCT01652911 | A Phase I/II Study of the Safety and Efficacy of Sernova's Cell PouchTM for Therapeutic Islet Transplantation | University of Alberta |
| NCT00784966 | Islet After Kidney Transplant for Type 1 Diabetes | Virginia Commonwealth University |
| NCT00790257 | Safety and Efficacy Study of Encapsulated Human Islets Allotransplantation to Treat Type 1 Diabetes | Cliniques universitaires Saint-Luc-Université Catholique de Louvain |
| NCT00853944 | Effect of Sitagliptin on Graft Function Following Islet Transplantation | University of British Columbia |
| NCT00249652 | Transplant and Addiction Project 1 | National Institute on Drug Abuse |
| NCT00530686 | Pancreatic Islet Cell Transplantation - A Novel Approach to Improve Islet Quality and Engraftment | Baylor Research Institute |
| NCT01123187 | Islet Cell Transplantation in Patients With Type 1 Diabetes With Previous Kidney Transplantation | University Hospital, Lille |
| NCT01817959 | Study to Assess Efficacy and Safety of Reparixin in Pancreatic Islet Transplantation | Dompé s.p.a. |
| NCT00679042 | Islet Transplantation in Type 1 Diabetic Patients Using the University of Illinois at Chicago Protocol | University of Illinois |
| NCT00453817 | Islet of Langerhans Graft Monitoring by Magnetic Resonance Imaging | University Hospital, Geneva |
| NCT00853424 | A Comparison of Islet Cell Transplantation With Medical Therapy for the Treatment of Diabetic Eye Disease | University of British Columbia |
| NCT00789308 | Safety and Effectiveness of Low Molecular Weight Sulfated Dextran in Islet Transplantation | National Institute of Allergy and Infectious Diseases |
| NCT01148680 | Trial Comparing Metabolic Efficiency of Islet Graft to Intensive Insulin Therapy for Type 1 Diabetes’s Treatment | University Hospital, Grenoble |
| NCT01241864 | Islet Transplantation in Type 1 Diabetic Kidney Allograft | University of Chicago |
| NCT01722682 | Bone Marrow vs Liver as Site for Islet Transplantation | Ospedale San Raffaele |
| NCT01630850 | Islet Transplantation in Patients With\Brittle\“Type 1 Diabetes” | University of Chicago |
| NCT01186562 | Sitagliptin Therapy to Improve Outcomes After Islet Autotransplant | University of Minnesota |
| NCT01285934 | A Trial of High Dose Immunosuppression and Autologous Hematopoietic Stem Cell Support Versus Intensive Insulin Therapy in Adults With Early Onset T1DM | University of Sao Paulo General Hospital |
| NCT00646724 | Cotransplantation of Islet and Mesenchymal Stem Cell in Type 1 Diabetic Patients | Fuzhou General Hospital |
| NCT01379729 | Bet Cell Therapy in Diabetes Type 1 | AZ-VUB |
| NCT01341899 | Efficacy and Safety Study of Autologous Hematopoietic Stem Cell Transplantation to Treat New Onset Type 1 Diabetes | The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School |
| NCT01736228 | Open-label Investigation of the Safety and Efficacy of DIABECELL in Patients With T1DM | Living Cell Technologies |
| NCT01346098 | Islet Autotransplantation in Patients at Very High-risk Pancreatic Anastomosis | Ospedale San Raffaele |
| NCT00989547 | Cord Blood Infusion for T1DM | Technische Universität München |
| NCT00807651 | Autologous Hematopoietic Stem Cell Transplantation for Early Onset Type 1 Diabetes | Shanghai Jiao Tong University School of Medicine |
| NCT01042301 | Profiling of Original Cellular and Humoral Biomarkers of Type 1 Diabetes | Nantes University Hospital |
| NCT01350219 | Stem Cell Educator Therapy in Type 1 Diabetes | Tianhe Stem Cell Biotechnologies Inc. |
| NCT00014911 | Immunosuppressive Medications for Participants in ITN005CT | National Institute of Allergy and Infectious Diseases |
It is apparent that in light of the therapeutic advantages of β-cell replacement through IT, numerous contributing factors hinder islet graft survival and function. These obstacles must be overcome in order for this therapy to become the ubiquitous alternative to pancreas transplantation and exogenous insulin administration. Despite intrahepatic islet infusion being the route of choice for over three decades[12,13] in both experimental and clinical settings, several complications with this approach exist which may account for islet graft attrition[14-18]. The liver indeed has an advantage of a multiple vascular supply, however, its parenchymal oxygen tension, is well below that of the pancreas and is not conducive to islet survival[19,20]. Furthermore, the infusion of islets into the liver is associated with inherit procedural risks including but not limited to catheter-induced hemorrhage and thrombosis[21]. Disadvantages of this route of islet administration also include limited ability to image islet grafts post-transplant, incapacity to retrieve the graft if required, and restricted quantity of β-cell mass that can recipient can receive due to portal pressure elevation[14-18,21,22]. The innate immune system further contributes to a reduction in β cells mass acutely post-infusion into the patient’s portal circulation. It is estimated that greater than 50% of the transplanted islets are lost within hours post infusion which is thought in part to be due to the immediate blood mediated inflammatory reaction and complement coagulation cascade, as evidenced by acute C-peptide release, and from quantitative positron emission tomography scan imagery[14-16,23-27]. These factors in conjunction with the diabetogenic action of the immunosuppressive drugs [i.e., calcineurin inhibits, sirolimus, mycophenolate mofetil (MMF)][28], suboptimal islet revascularization[29,30], both the adaptive and innate immune responses, potential HLA-antigen[31-34] sensitization and lack of an effective means of determining islet potency prior to transplant, together contribute to an inept utilization of the small number of available cadaveric donor pancreata[16,17]. The difficulties and limitations associated with hepatic portal vein infusion have stimulated robust efforts into investigation strategies to improve islet engraftment, such as refined IS protocols, surrogate sources of β-cells (i.e., stem cells or porcine islets) and alternative transplantation sites, in effort to increase the potential for long-term islet graft survival and function[16,18].
The early results from IT should be taken into context and compared to alternative treatments modalities. In contrast to pancreas transplantation, IT is still in its infancy. Roughly 750 type 1 diabetic patients have received an IT among the some 30 active international islet centers over the past decade. In comparison, approximately 30000 pancreas transplants have been conducted over the past three decades[35-38]. Despite the relatively low number of islet recipients, encouraging results with IT have recently been reported, such as the greater than 50% insulin-independent rates from solitary pancreas transplantation 5 years post-transplant has now been matched by IT in at least four independent centers, namely Edmonton, Minneapolis, Genva and Lille. It is evident that recent significant advances in islet preparation and immunosuppressive therapy have improved the efficacy and safety of IT to the point that it now challenges whole organ pancreas transplantation.
Due to the multiple pathways known to be involved in β-cell attrition, including the autoreactive and alloreactive immune response, as well as the alloresponse it can be argued that a monotherapy IS approach is improbable to further enhance IT outcomes. Indeed, strategies towards single-donor IT has begun by implementing multiple pathways blockades to IS cocktails, which face the challenges of promoting islet graft survival. Combining anti-inflammatory biologics to maintenance IS have led to improved single-donor success rates at the University of Minnesota[39,40]. The success rate of islet donor islet recipients has dramatically increased from 10%-40% when peritransplant insulin and heparin intervention has been employed[27]. Tumor necrosis factor-α blockage by etanercept has improved single-donor islet transplant outcomes as well[27,40-44]. In preclinical settings specific anti-inflammatory agents such as the interleukin-1 receptor antagonist anakinra and etanercept significantly increased marginal mass islet engraftment[41-45]. Furthermore, anti-apoptosis and growth stimulation [i.e., glucagon-like peptide 1 (GLP-1)] have further demonstrated advantageous results in both preclinical and clinical studies, for instance the short acting GLP-1 analogue exenatide demonstrated an increased single-donor islet engraftment success rate[46-48]. Clonal depletion of alloreactive T cells appears promote a hyporesponsive environment and peripheral mechanisms of anergy, thus driving the shift towards tolerance[49,50]. The use of T-cell depletion induction methods such as alemtuzumab in conjunction with TAC/MMF have resulted in substantial improvements in long-term insulin-independence (> 5 years)[51,52]. In addition, a current example of the extraordinary progress that has been made when combine IS strategies are implemented, is the remarkable success that has been achieved when co-stimulation blockage using belatacept (inhibiting CD80-CD86 interactions) in conjunction with T-cell depletion induction and in the absence of calcineurin inhibitors led to insulin independence with islets from a single donor and prolonged allograft survival[6,53]. It is clear current immunosuppressive therapies have become well tolerated and safer for the recipient by minimizing the adverse side effects while improving islet engraftment.
Since the first pioneering experimental and clinical studies, substantial improvements have been made in IT, leading to the development of the “Edmonton Protocol”. Over the past decade, since this protocols inception, continued progress in the field has resulted in markedly higher rates of single-islet recipient success rates as well as sustained insulin-independences (> 5 years). Not to be forgotten are the benefits for microvascular complications (i.e., reduced retinopathy) and the amelioration of hypoglycemic unaware events following IT, in most cases irrespective of glycemic control. Despite the favourable long-term safety profile associated with IT, many unanswered questions still exist; namely, the causality of islet graft function and attrition. For instance reduction in HbA1C and hypoglycaemia normally attributed to graft function may in part be a reflection of close glycemic monitoring. Equally, graft dysfunction and poor glycemic control post-transplant may be attributed to poor adherence and psychosocial influences among others, rather than exclusively caused by islet graft loss[54]. Some of these answers may very well indeed be answered through randomized clinical trials. By no means should IT be perceived as a cure for all type 1 diabetics, however for a subset of individuals with severe glycemic lability, IT has been demonstrated to be an excellent therapeutic strategy to achieve glycemic control and abrogation of hypoglycaemia. As the field of continues to work and progress together, in effort to refine and optimized IT, it is foreseeable that a cure for T1DM is obtainable in the not so distant future.
P- Reviewers: Chaib E, Fulop T S- Editor: Qi Y L- Editor: A E- Editor: Zheng XM
| 1. | Ryan EA, Bigam D, Shapiro AM. Current indications for pancreas or islet transplant. Diabetes Obes Metab. 2006;8:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1480] [Cited by in RCA: 1422] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 3. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3825] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 4. | Jahansouz C, Jahansouz C, Kumer SC, Brayman KL. Evolution of β-cell replacement therapy in diabetes mellitus: Islet cell transplantation. J Transplant. 2011;2011:247959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Shapiro J, Shaw J. A historical perspective on experimental and clinical islet transplantation. Islet transplantation and beta cell replacement therapy. New York: Informa Healthcare 2007; 1-28. |
| 6. | Gala-Lopez B, Pepper AR, Shapiro AM. Biologic agents in islet transplantation. Curr Diab Rep. 2013;13:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Squifflet JP, Gruessner RW, Sutherland DE. The history of pancreas transplantation: past, present and future. Acta Chir Belg. 2008;108:367-378. [PubMed] |
| 8. | Han DJ, Sutherland DE. Pancreas transplantation. Gut Liver. 2010;4:450-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 271] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060-2069. [PubMed] |
| 11. | Senior PA, Kin T, Shapiro J, Koh A. Islet transplantation at the University of Alberta: Status update and review of progress over the last decade. Can J Diabetes. 2012;36:32-37. |
| 12. | Scharp DW, Murphy JJ, Newton WT, Ballinger WF, Lacy PE. Transplantation of islets of Langerhans in diabetic rhesus monkeys. Surgery. 1975;77:100-105. [PubMed] |
| 13. | Kemp CB, Scharp DW, Knight MJ, Ballinger WF, Lacy PE. Importance of implantation site of pancreatic islet isografts in treatment of experimental diabetes. Surg Forum. 1973;24:297-299. [PubMed] |
| 14. | Korsgren O, Nilsson B. Improving islet transplantation: a road map for a widespread application for the cure of persons with type I diabetes. Curr Opin Organ Transplant. 2009;14:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Korsgren O, Lundgren T, Felldin M, Foss A, Isaksson B, Permert J, Persson NH, Rafael E, Rydén M, Salmela K. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia. 2008;51:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Harlan DM, Kenyon NS, Korsgren O, Roep BO. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest. 2004;114:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Merani S, Toso C, Emamaullee J, Shapiro AM. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95:1449-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Carlsson PO, Palm F, Andersson A, Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation. 2000;69:761-766. [PubMed] |
| 20. | Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489-495. [PubMed] |
| 21. | Kawahara T, Kin T, Kashkoush S, Gala-Lopez B, Bigam DL, Kneteman NM, Koh A, Senior PA, Shapiro AM. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am J Transplant. 2011;11:2700-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Kawahara T, Kin T, Shapiro AM. A comparison of islet autotransplantation with allotransplantation and factors elevating acute portal pressure in clinical islet transplantation. J Hepatobiliary Pancreat Sci. 2012;19:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907-1914. [PubMed] |
| 24. | Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105:125-133. [PubMed] |
| 25. | Eriksson O, Eich T, Sundin A, Tibell A, Tufveson G, Andersson H, Felldin M, Foss A, Kyllönen L, Langstrom B. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9:2816-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Eich T, Eriksson O, Lundgren T. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med. 2007;356:2754-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Koh A, Senior P, Salam A, Kin T, Imes S, Dinyari P, Malcolm A, Toso C, Nilsson B, Korsgren O. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, Tee M, Potter KJ, Klimek AM, Meloche RM. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18:833-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318-1325. [PubMed] |
| 30. | Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008;57:2269-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, Imes S, Halpin A, Ryan EA, Shapiro AM. High risk of sensitization after failed islet transplantation. Am J Transplant. 2007;7:2311-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Campbell PM, Salam A, Ryan EA, Senior P, Paty BW, Bigam D, McCready T, Halpin A, Imes S, Al Saif F. Pretransplant HLA antibodies are associated with reduced graft survival after clinical islet transplantation. Am J Transplant. 2007;7:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Naziruddin B, Wease S, Stablein D, Barton FB, Berney T, Rickels MR, Alejandro R. HLA class I sensitization in islet transplant recipients: report from the Collaborative Islet Transplant Registry. Cell Transplant. 2012;21:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Jackson AM, Connolly JE, Matsumoto S, Noguchi H, Onaca N, Levy MF, Naziruddin B. Evidence for Induced Expression of HLA Class II on Human Islets: Possible Mechanism for HLA Sensitization in Transplant Recipients. Transplantation. 2009;87:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep. 2011;11:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Kandaswamy R, Sutherland DE. Pancreas versus islet transplantation in diabetes mellitus: How to allocate deceased donor pancreata? Transplant Proc. 2006;38:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463-501. [PubMed] |
| 38. | Sutherland DE, Gruessner RW, Gruessner AC. Pancreas transplantation for treatment of diabetes mellitus. World J Surg. 2001;25:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Hering BJ. Repurification: rescue rather than routine remedy. Am J Transplant. 2005;5:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 41. | Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, Itoh T, Chujo D, SoRelle J, Onaca N. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant. 2011;20:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Shapiro AM, Ricordi C. Unraveling the secrets of single donor success in islet transplantation. Am J Transplant. 2004;4:295-298. [PubMed] |
| 43. | Xenos ES, Farney AC, Widmer MB, Casanova D, Stevens RB, Blazar BR, Sutherland DE, Gores PF. Effect of tumor necrosis factor alpha and of the soluble tumor necrosis factor receptor on insulin secretion of isolated islets of Langerhans. Transplant Proc. 1992;24:2863-2864. [PubMed] |
| 44. | Rewers M, Gottlieb P. Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future. Diabetes Care. 2009;32:1769-1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant. 2012;12:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Cechin SR, Pérez-Álvarez I, Fenjves E, Molano RD, Pileggi A, Berggren PO, Ricordi C, Pastori RL. Anti-inflammatory properties of exenatide in human pancreatic islets. Cell Transplant. 2012;21:633-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Faradji RN, Froud T, Messinger S, Monroy K, Pileggi A, Mineo D, Tharavanij T, Mendez AJ, Ricordi C, Alejandro R. Long-term metabolic and hormonal effects of exenatide on islet transplant recipients with allograft dysfunction. Cell Transplant. 2009;18:1247-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, Warnock GL, Elahi D, Meneilly GS, Thompson DM. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation. 2007;83:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Bhatt S, Fung JJ, Lu L, Qian S. Tolerance-inducing strategies in islet transplantation. Int J Endocrinol. 2012;2012:396524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, Chinnakotla S, Vickers SM, Bland B, Balamurugan AN. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409-424; discussion 424-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 51. | Shapiro AM. Strategies toward single-donor islets of Langerhans transplantation. Curr Opin Organ Transplant. 2011;16:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Froud T, Baidal DA, Faradji R, Cure P, Mineo D, Selvaggi G, Kenyon NS, Ricordi C, Alejandro R. Islet transplantation with alemtuzumab induction and calcineurin-free maintenance immunosuppression results in improved short- and long-term outcomes. Transplantation. 2008;86:1695-1701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Posselt AM, Szot GL, Frassetto LA, Masharani U, Tavakol M, Amin R, McElroy J, Ramos MD, Kerlan RK, Fong L. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90:1595-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 535] [Article Influence: 41.2] [Reference Citation Analysis (0)] |