Published online Jan 18, 2023. doi: 10.5500/wjt.v13.i1.10
Peer-review started: October 7, 2022
First decision: November 14, 2022
Revised: November 24, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 18, 2023
Despite the increased use of total pancreatectomy with islet autotransplantation (TPIAT), systematic evidence of its outcomes remains limited.
To evaluate the outcomes of TPIAT.
We searched PubMed, EMBASE, and Cochrane databases from inception through March 2019 for studies on TPIAT outcomes. Data were extracted and analyzed using comprehensive meta-analysis software. The random-effects model was used for all variables. Heterogeneity was assessed using the I2 measure and Cochrane Q-statistic. Publication bias was assessed using Egger’s test.
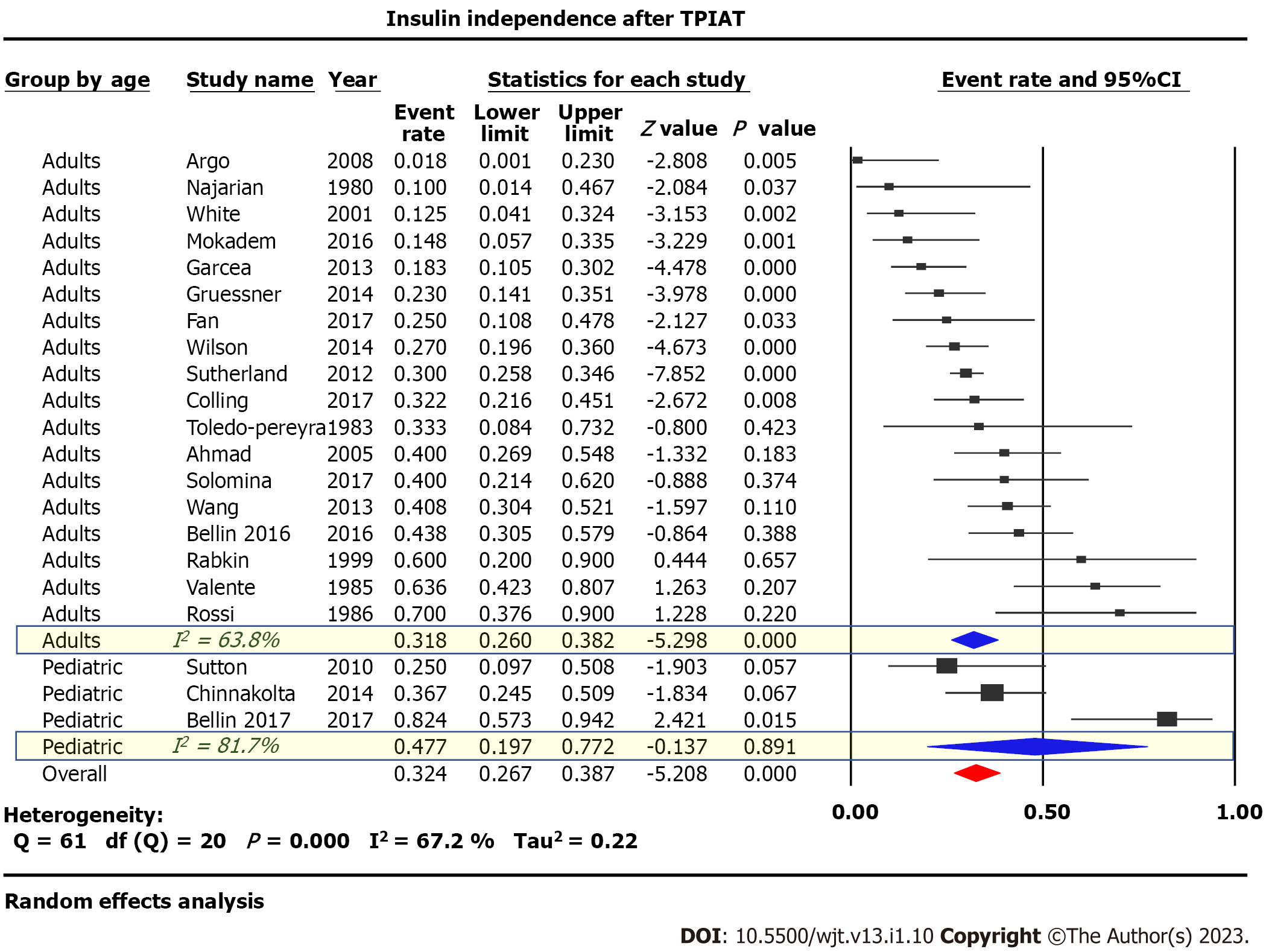
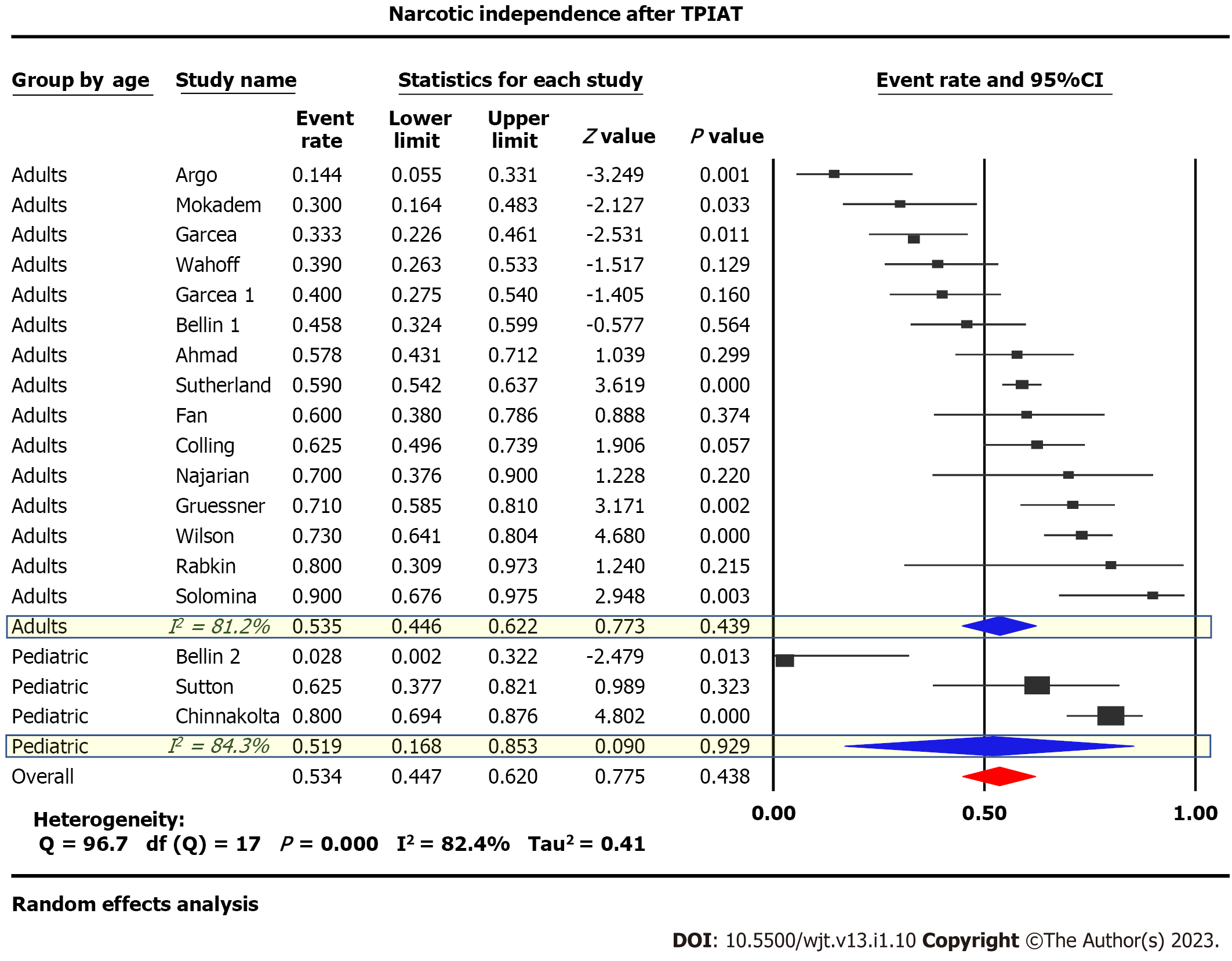
Twenty-one studies published between 1980 and 2017 examining 1011 patients were included. Eighteen studies were of adults, while three studied pediatric populations. Narcotic independence was achieved in 53.5% [95% Confidence Interval (CI): 45-62, P < 0.05, I2 = 81%] of adults compared to 51.9% (95%CI: 17-85, P < 0.05, I2 = 84%) of children. Insulin-independence post-procedure was achieved in 31.8% (95%CI: 26-38, P < 0.05, I2 = 64%) of adults with considerable heterogeneity compared to 47.7% (95%CI: 20-77, P < 0.05, I2 = 82%) in children. Glycated hemoglobin (HbA1C) 12 mo post-surgery was reported in four studies with a pooled value of 6.76% (P = 0.27). Neither stratification by age of the studied population nor meta-regression analysis considering both the study publication date and the islet-cell-equivalent/kg weight explained the marked heterogeneity between studies.
These results indicate acceptable success for TPIAT. Future studies should evaluate the discussed measures before and after surgery for comparison.
Core Tip: Surgical intervention is required for the management of debilitating and refractory abdominal pain in chronic pancreatitis (CP) patients failing medical therapy. Since first introduced in 1978, total pancreatectomy with islet autotransplantation (TPIAT) has shown promising results in CP patients, but the literature remains limited. This systematic review and meta-analysis found that TPIAT provided acceptable levels of pain relief and insulin independence.
- Citation: Khazaaleh S, Babar S, Alomari M, Imam Z, Chadalavada P, Gonzalez AJ, Kurdi BE. Outcomes of total pancreatectomy with islet autotransplantation: A systematic review and meta-analysis. World J Transplant 2023; 13(1): 10-24
- URL: https://www.wjgnet.com/2220-3230/full/v13/i1/10.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i1.10
Chronic pancreatitis (CP) is characterized by progressive inflammation of the pancreas with eventual fibrosis, ductal alteration, and permanent structural damage. CP has a reported mortality of nearly 50% within the first 20-25 years of diagnosis[1,2]. It significantly impairs the quality of life (QoL) of the affected patients, often requiring frequent Emergency Department (ED) visits and hospitalizations due to pain, infections, malnutrition, and recurrent acute on chronic pancreatitis[3]. The clinical manifestations include varying degrees of abdominal pain, malabsorption from exocrine insufficiency, and the development of diabetes mellitus (DM). Although the compromised exocrine function and DM can be treated with oral pancreatic enzyme supplementation and insulin, the hallmark symptom of CP is pain, which often is intractable and debilitating[4].
The commonly used first-line therapies for CP primarily focus on mitigating the unrelenting and recurring abdominal pain. These include dietary modifications with a low-fat diet, pancreatic enzyme supplementation, strict smoking cessation, and alcohol abstinence[5]. Despite these initial measures, many patients often end up requiring frequent escalating doses of narcotics with consequent opioid dependence[6]. Patients who require chronic opioids are often candidates for invasive procedures in an attempt to eliminate or modify the underlying source of pain[7]. Frequently, endoscopic treatments such as sphincterotomy and/or stent placement are employed to treat fibrotic strictures of the pancreatic duct or stone extraction if present[8,9]. When the usual medical and endoscopic therapies fail to address the severe pain and subsequent life disruption, surgical treatments, including functional operative diversion (i.e., pancreatojejunostomy) or operative gland extirpation (i.e., pancreatectomy), are advocated depending on the pancreatic ductal and parenchymal anatomy.
A recent randomized control trial (RCT) demonstrated that surgical approaches are more effective at eliminating pain and have more extended durability, thus reducing the need for repeated interventions when compared to endoscopic therapies. The creation of a longitudinal pancreatojejunostomy in functional diversion alleviates some of the exocrine insufficiency in CP; however, the retained native gland often leads to the recurrence of chronic pain and subsequent treatment failure. This pitfall also applies to the other types of partial pancreatectomies, such as the isolated resection of the pancreatic head (with or without duodenal preservation) or resection of the body/tail of the pancreas.
Total pancreatectomy (TP), which involves the excision of the entire gland, is often successful in eradicating the underlying cause of pain in CP. TP has historically been avoided due to the heightened risk of exocrine dysfunction and the difficulty in managing the brittle endocrine dysfunction associated with this procedure[10]. Subsequently, TP with islet autotransplantation (TPIAT) was introduced for the management of CP[11]. This procedure involves complete resection of the pancreas with trans portal islet cell transplantation (IAT)[12]. This comprehensive procedure has been postulated to eliminate the visceral source of pain along with a reduced risk of post-surgical DM. The use of concomitant IAT has been demonstrated to reduce or eliminate the need for exogenous insulin administration after a TP in many modern studies[13-16]. TPIAT has been reported to be more cost-effective than the medical management of CP in a recent single-center cost analysis. While it was initially recommended for adult patients with long-standing pancreatitis, TPIAT is now also being utilized in pediatric patients with CP and even in adults with intractable acute recurrent pancreatitis[17,18]. Although the open approach remains the standard, this surgical procedure has evolved over time, with some centers offering minimally invasive laparoscopic operative options.
Despite the emerging popularity of TPIAT for CP, the available data on the appropriate indications, procedural technique, and short and long-term outcomes-such as narcotic dependence and deve
We performed a comprehensive literature search in PubMed, EMBASE, and Cochrane databases from inception through March 2019, to identify all studies that evaluated post-procedural insulin or narcotic independence rates after TPIAT. We used the following keywords in different combinations for our search: Pancreatectomy, pancreatic resection, islet, autotransplantation, chronic, pancreatitis, insulin independence, narcotic independence, pain, outcome, and diabetes. The search was limited to human studies with no restrictions placed on region, publication type, or language. References of all included studies were manually searched for additional eligible papers.
Two authors independently performed the literature review (SB and BE). The data from the included studies were entered into a standardized table for analysis. To be included, studies were required to meet the following criteria: (1) Implemented a well-defined RCT, case-control, cohort, or case-series design; and (2) either presented an odds ratio (OR) for our main outcomes with a 95% confidence interval (CI) or presented the data sufficient to calculate the OR with a 95%CI. Studies were excluded if they provided insufficient information to calculate the OR for narcotic independence, insulin-independence, or HbA1C levels 12 mo post-surgery. Studies were excluded if they were letters to editors, case reports, or review articles.
The quality of included studies was assessed independently by two of the authors (ZI and BE) using the Newcastle-Ottawa scale for cohort studies (Table 1) and the Murad tool for case series (Table 2), respectively[19,20]. Case series were considered of good methodological quality if they reported adequately on the domains of selection, exposure, outcome, and follow-up. Two authors (ZI and BE) addressed the discrepancies by joint evaluation of the original article.
| Ref. | Publish year | Study design | Q11 | Q22 | Q33 | Q44 | Q55 | Q66 | Q77 | Q88 | Total |
| Adult cohorts | |||||||||||
| Argo et al[39] | 2008 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Najarian et al[30] | 1980 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| White et al[31] | 2001 | Cohort, P | * | * | * | * | * | * | * | * | ********(8) |
| Mokadem et al[32] | 2016 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Garcae et al[24] | 2013 | Cohort, P | * | * | * | * | * | * | * | *******(7) | |
| Gruessner et al[33] | 2014 | Cohort, P | * | * | * | * | * | * | ******(6) | ||
| Wilson et al[15] | 2014 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Sutherland et al[16] | 2012 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Colling et al[35] | 2017 | Cohort, R | * | * | * | * | ** | * | * | * | *********(9) |
| Ahmad et al[29] | 2005 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Solomina et al[25] | 2017 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Wang et al[36] | 2013 | Cohort, R | * | * | * | * | * | * | *******(7) | ||
| Bellin et al[18] | 2016 | Cohort, P | * | * | * | * | * | * | ******(6) | ||
| Rabkin et al[37] | 1999 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Valente et al[41] | 1985 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Wahoff et al[50] | 1995 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Garcea et al[51] | 2009 | Cohort, P | * | * | * | * | * | * | * | *******(7) | |
| Pediatric cohorts | |||||||||||
| Sutton et al[44] | 2010 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Chinnakotla et al[17] | 2014 | Cohort, R | * | * | * | * | * | * | ******(6) | ||
| Bellin et al[18] | 2016 | Cohort, R | * | * | * | * | * | * | * | *******(7) | |
| Bellin et al[52] | 2010 | Cohort, R | * | * | * | * | * | *****(5) |
Statistical analysis was performed using the Comprehensive Meta-Analysis (CMA), Version 3 software (BioStat, Inc., Englewood, NJ, United States). Effect estimates from the individual studies were extracted and combined using the random-effect, generic inverse variance method of DerSimonian and Laird[21]. A random effect model was used as a high probability of between-study variance was suspected due to variation in the study population and methodology. A pooled OR was calculated. A Cochran’s Q-test and an I2 statistic were used to evaluate heterogeneity and quantify variation across the selected studies[22]. A funnel plot was then created to evaluate for publication and other reporting biases and then the plot was examined visually for asymmetry. Then, an Egger test for the asymmetry of a funnel plot was conducted. All authors had access to the study data and reviewed and approved the final manuscript.
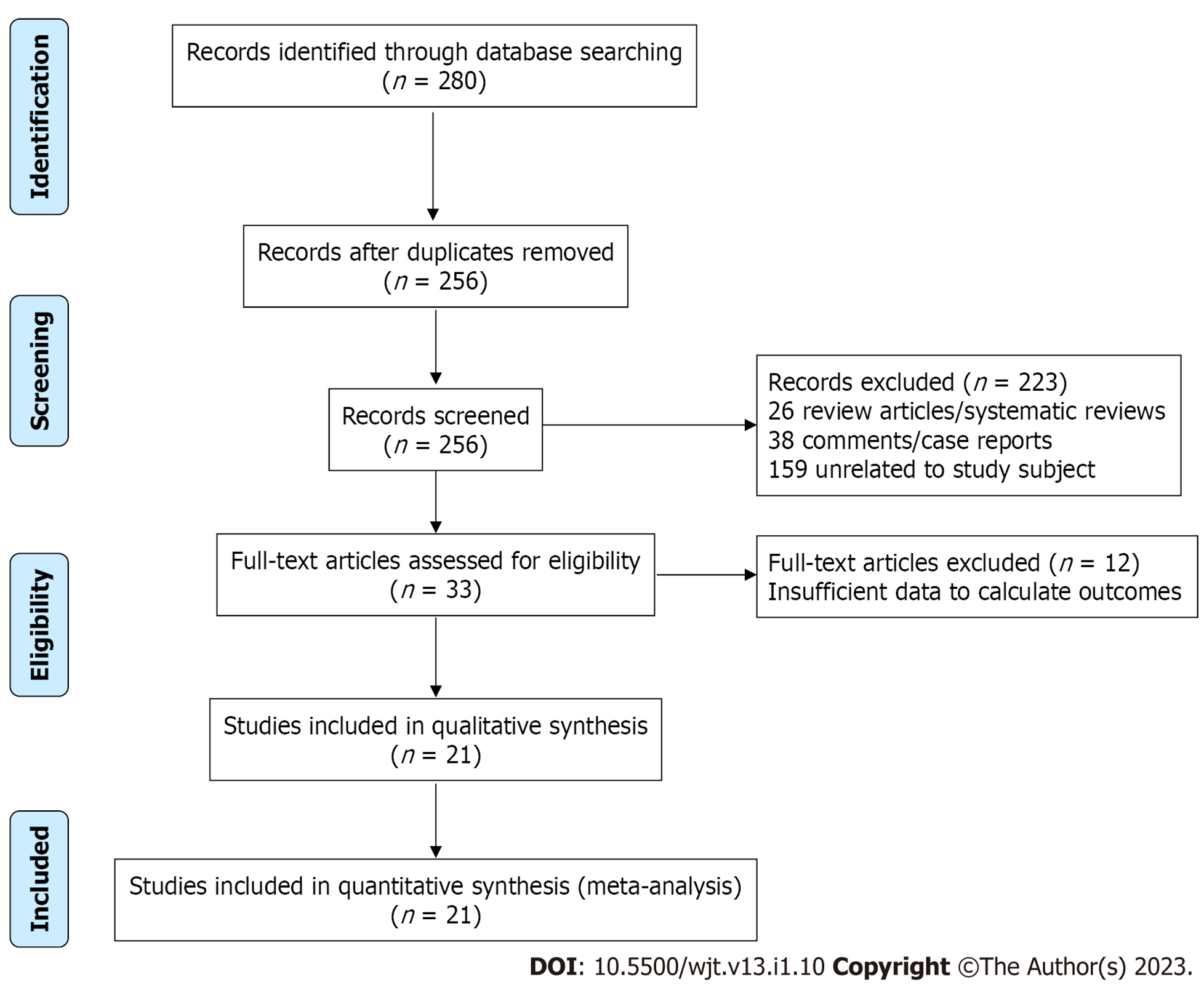
Our initial comprehensive search yielded 280 citations. All citations underwent a title and abstract review, with the majority being excluded as duplicates, letters to editors, case reports, review articles, or unrelated to the study subject. Of our initial yield, 33 citations underwent a full-length article review. Of these, 12 were excluded as review articles or did not provide sufficient information to calculate post-procedural insulin or narcotic independence rates in the studied populations. A flow diagram illustrates the selection process, in Figure 1. Consequently, a total of 21 studies met our inclusion criteria and were included in the meta-analysis. Published between 1980 and 2017, these papers included 1011 patients. Eighteen papers reviewed adult populations, while three studied pediatric populations (SM2). The baseline characteristics of the included studies and involved cohorts are summarized in Tables 3 and 4.
| Ref. | Study design | Data collected | Year published | Participants enrolled, n | Patients underwent TPIAT, n | Age, mean (SD)/range, yr | Female sex, n (%) |
| Adults | |||||||
| Argo et al[39] | Retrospective cohort | 2005-2007 | 2008 | 26 | 26 | 43.8 (2.1) | 12 (46) |
| Najarian et al[30] | Retrospective cohort | 1977-1980 | 1980 | 18 | 10 | 24-57 | 4 (40) |
| White et al[31] | Prospective cohort study | 1994-1999 | 2001 | 37 | 24 | 44 (NA) | 14 (58) |
| Mokadem et al[32] | Retrospective cohort | 1998-2008 | 2016 | 70 | 57 | 39.9 (14) | 32 (56) |
| Garcae et al[24] | Prospective cohort study | 1990-2012 | 2013 | 97 | 60 | 43 (NA) 21-65 | Unknown |
| Gruessner et al[33] | Prospective cohort study | 2009-2013 | 2014 | 61 | 61 | 42.2 (1.6) | 39 (64) |
| Fan et al[34] | Case series | 2013-2015 | 2017 | 32 | 20 | 39 (13) 21-58 | 12 (60) |
| Wilson et al[15] | Retrospective cohort | 2000-2013 | 2014 | 166 | 166 | 37.3 (1.1) 14-62 | 75 (67) |
| Sutherland et al[16] | Retrospective cohort | 1977-2011 | 2012 | 409 | 4091 | 35.3 (0.7) 5-69 | 301 (74) |
| Colling et al[35] | Retrospective cohort | 2002- 2014 | 2017 | 59 | 59 | Unknown | 30 (51) |
| Toledo-Pereyra et al[40] | Case series | 1979-1981 | 1983 | 6 | 6 | 35.5 (6.0) 28-41 | 1 (17) |
| Ahmad et al[29] | Retrospective cohort | 2000-2004 | 2005 | 45 | 45 | 39 (NA) 16-62 | 30 (67) |
| Solomina et al[25] | Retrospective cohort | unknown | 2017 | 20 | 20 | 41 (NA) 15-60 | 13 (65) |
| Wang et al[36] | Retrospective cohort | 2009-2011 | 2013 | 76 | 76 | 42.1 (11.4) | Unknown |
| Bellin et al[18] | Retrospective cohort | 2007-2013 | 2016 | 49 | 49 | 32.8 (7.8) | 42 (86) |
| Rabkin et al[37] | Retrospective cohort | 1994-1997 | 1999 | 5 | 5 | 42 ( NA) | 4 (80) |
| Valente et al[41] | Retrospective cohort | unknown | 1985 | 25 | 22 | Unknown | Unknown |
| Rossi et al[38] | Case series | 1981-1985 | 1986 | 10 | 10 | 34 (NA) 23-65 | 6 (60) |
| Wahoff et al[50] | Retrospective cohort | 1977-1995 | 1995 | 48 | 48 | 35 (NA) 12-60 | 36 (75) |
| Garcea et al[51] | Prospective cohort study | 1996-2006 | 2009 | 85 | 50 | 43 (NA) 21-65 | 26 (52) |
| Pediatrics | |||||||
| Sutton et al[44] | Retrospective cohort | 2000-2009 | 2010 | 188 | 118 | 31.4 (NA) 15-59 | 8 (50) |
| Chinnakotla et al[17] | Retrospective cohort | 1989-2012 | 2014 | 75 | 75 | 13.8 (0.4) | 42 (56) |
| Bellin et al[18] | Retrospective cohort | 2000-2014 | 2016 | 17 | 17 | 6.8 (NA) | 9 (53) |
| Bellin et al[52] | Retrospective cohort | 1989-2006 | 2010 | 18 | 18 | 12.8 (4.08) 5.8-18.9 | 10 (55.6) |
| Ref. | Pre-operative diabetes, n (%) | Alcohol induced pancreatitis, n (%) | Biliary tract disease, n (%) | Idiopathic pancreatitis, n (%) | Genetic mutation, n (%) | Pancreatic divism, n (%) | Autoimmune pancreatitis, n (%) | Post-operative narcotic independence, n (%) | Post-operative insulin independence, n (%) | Mean percent glycosylated hga1c, %, (SD), range |
| Adults | ||||||||||
| Argo et al[39] | Unknown | 9 (35) | 1 (4) | 8 (31) | 0 | 6 (23) | 0 | 3 (60) | 0 (0) | |
| Najarian et al[30] | 0 (0) | 6 (60) | 1 (10) | 3 (30) | 1 (10)1 | 0 (0) | 0 (0) | 7 (78) | 4 (40) at range 1-38 mo | |
| White et al[31] | 0 (0) | 8 (18) | 2 (5) | 13 (30)2 | 0 (0) | 1 (2) | 0 (0) | 16 (77) | 8 (33) transient/3 (13) at writing | |
| Mokadem et al[32] | 0 (0) | 4 (7) | 2 (4) | 19 (63) | 0 (0) | 5 (17) | 0 (0) | 9 (16) | 4 (15) | |
| Garcae et al[24] | Unknown | 19 (32) | 5 (8) | 31 (52) | 0 (0) | 0 (0) | 0 (0) | 27 (45) | 11 (19) | |
| Gruessner et al[33] | Unknown | 7 (11) | 0 (0) | 45 (73) | 10 (16) | 0 (0) | 0 (0) | 43 (71) | 12 (19) at range 1-24 mo | |
| Fan et al[34] | Unknown | 2 (10) | 0 (0) | 6 (30) | 9 (45) | 3 (15) | 0 (0) | 12 (60) at 6 mo | 5 (25) at 12.5 mo | 7.4 (0.5) |
| Wilson et al[15] | 14 (13) | 3 (3) | 0 (0) | 84 (75) | 15 (13) | 10 (9) | 0 (0) | 91 (55) at 1 yr /121 (73) at 5 yr | 62 (38) at 1 yr/45 (27) at 5 yr | 6.9 (0.3) 5.85-8.3 |
| Sutherland et al[16] | 32 (8) | 27 (7) | 36 (9) | 169 (41) | 58 (14) | 71 (17) | 0 (0) | 241 (59) at 2 yr3 | 123 (30) at 3 yr4 | |
| Colling et al[35] | 3 (5) | 0 (0) | 2 (3) | 6 (10) | 49 (83) | 4 (7) | 0 (0) | 35 (66) at 1 yr | 19 (32) at 1 yr | |
| Toledo-Pereyra et al[40] | 0 (0) | 3 (50) | 0 (0) | 3 (50) | 0 (0) | 0 (0) | 0 (0) | 2 (50) at 20 and 25 mo | ||
| Ahmad et al[29] | 1 (2) | 2 (4) | 0 (0) | 39 (87) | 1 (2) | 8 (18) | 0 (0) | 23 (72) at 5 mo | 18 (40) at mean 18 mo | |
| Solomina et al[25] | Unknown | 0 (0) | 0 (0) | 3 (15) | 13 (65) | 3 (15) | 1 (5) | 18 (87) at 1 yr | 8 (53) at 1 yr | |
| Wang et al[36] | 11 (14) | Unknown | Unknown | Unknown | Unknown | Unknown | 0 (0) | NA | 31 (41) at 6 mo | |
| Bellin et al[18] | 2 (4) | 0 (0) | 13 (27) | 18 (37) | 4 (8) | 11 (22) | 0 (0) | 22 (46) at 1 yr | 21 (45) at 1 yr | 6.0 (0.9) at 1 yr |
| Rabkin et al[37] | 0 (0) | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | 3 (60) | 4 (80) | 3 (60) at median 23 mo | 6.43 (1.50) 5.1-8.0 |
| Valente et al[41] | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | 14 (64) at mean 5 yr | |
| Rossi et al[38] | Unknown | 2 (20) | 0 (0) | 8 (80) | 0 (0) | 1 (10) | 0 (0) | 9 (90) | 7 (70) at 2 yr | |
| Wahoff et al[50] | 2 (4) | 9 (19) | 8 (16) | 27 (56) | 0 (0) | 2 (4) | 0 (0) | 31 (81) | 13 (34) | |
| Garcea et al[51] | 0 (0) | 18 (36) | 5 (10) | 24 (48) | 0 (0) | 0 (0) | 0 (0) | 30 (59.8) at 1 yr | ||
| Pediatrics | ||||||||||
| Sutton et al[44] | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (100) | 0 (0) | 0 (0) | 10 (63) at mean 22 mo | 4 (25) at mean 22 mo | |
| Chinnakotla et al[17] | Unknown | 0 (0) | 0 (0) | 21 (28) | 41 (55) | 0 (0) | 0 (0) | 13(17) | 31 (41) | |
| Bellin et al[18] | 0 (0) | 0 (0) | 0 (0) | 2 (12) | 14 (82) | 1 (6) | 0 (0) | 17 (100) | 14 (82) | |
| Bellin et al[52] | 0 (0) | 0(0) | 1 (6) | 7 (39) | 7 (39) | 3 (17) | 0 (0) | 11 (61) at median 2.5 (0.2-17.1) | 11 (61) at 1 year or longer | 6.40 (2.34) 5.0-12.5 at 4.5 (5.2); n = 8 |
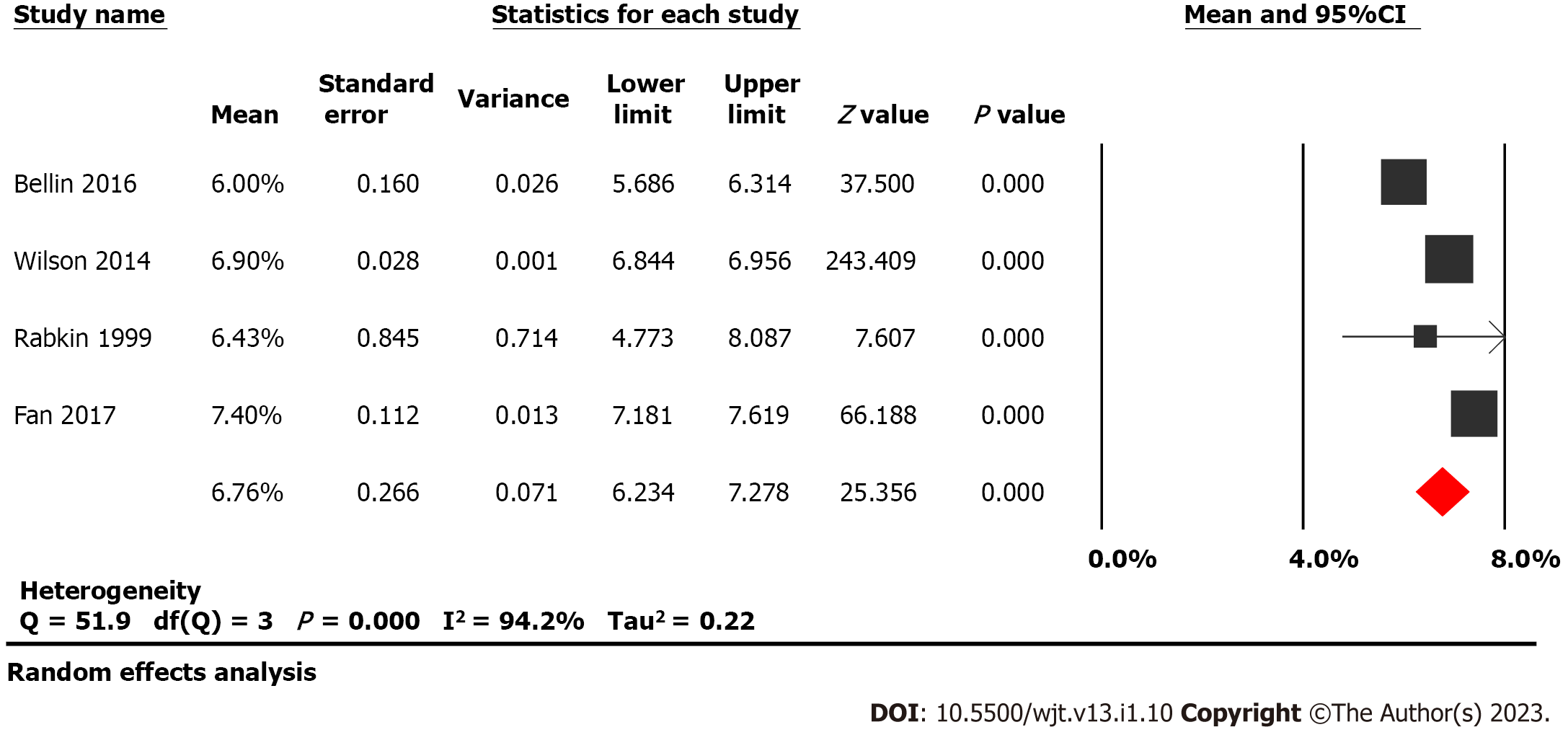
Twenty-one studies examining 1011 patients were included in this study. Insulin-independence post-procedure was achieved in 31.8% (95%CI: 26-38, P < 0.05, I2 = 64%) of adults compared to 47.7% (95%CI: 20-77, P < 0.05, I2 = 82%) of children, Figure 2. Narcotic independence was achieved in 53.5% (95%CI: 45-62, P < 0.05, I2 = 81%) of adults compared to 51.9% (95%CI: 17-85, P < 0.05, I2 = 84%) of children (Figure 3). Glycated hemoglobin (HbA1C) 12 mo post-surgery was reported in four studies evaluating adult populations with a pooled value of 6.76% (P = 0.27) (Figure 4).
Funnel plots were generated to evaluate post-procedural insulin and narcotic independence. The plots are symmetric and do not suggest the presence of publication bias. Egger’s regression asymmetry testing was also done to demonstrate no evidence of publication bias (P > 0.05).
Neither stratification by age of the studied population nor meta-regression analysis considering both the study publication date and the islet-cell-equivalent/kg weight were able to explain the marked heterogeneity between studies.
When persistent abdominal pain in patients with CP becomes debilitating and the best medical management cannot stop the intractable pain, surgical intervention is indicated. Since first described by Sutherland et al[23] in 1978, TPIAT has shown promising results for patients with CP. Sutherland et al[23] hypothesized that by combining TP with IAT, TPIAT removes the primary pain source while maintaining endocrine function. TPIAT preserves insulin-secreting capacity and avoids post-surgical DM through the conservation of beta cell mass and C-peptide positivity[23]. In the years following the first-performed TPIATs, the procedure is being increasingly used for patients with CP and intractable pain[24]. QoL metrics show TPIAT as equal or superior to traditional TPs[24-27]. Morbidity and mortality metrics also support TPIAT as a safe and feasible procedure[28]. However, over this same time, minimal systematic evidence has been collected on metabolic function and pain control following TPIAT. In this paper, we present the most current meta-analysis to date and a systemic review of literature on insulin and narcotic independence after TPIAT.
Our study examined 1011 patients across 21 studies and found that 31.8% of adults were insulin independent after TPIAT[15,16,18,24,25,29-41]. Additionally, many patients who were not insulin-independent following TPIAT required only minimal amounts of exogenous insulin to achieve blood sugar control. HbA1c is 6.76% 12 mo post-surgery in four studies of 240 adult patients[15,18,34,37]. In total, these studies describe populations that vary by age, sex, and disease etiology. Data were collected on patients from two countries and nearly four decades to present the largest known meta-analysis to date on this topic.
Our analysis also reviewed insulin and narcotic independence after TPIAT in pediatric patients. The first TPIAT performed on a pediatric patient occurred in 1996[42]. Since, several authors have reviewed QoL, morbidity, and mortality metrics in this particular patient population. We identified studies that have reviewed insulin dependence after TPIAT in pediatric populations, totaling 181 patients[16,17,43,44]. Our research found 47.7% of children were insulin-independent post-TPIAT.
The majority of TPIATs were performed on patients with idiopathic CP (49.10%). Other common etiologies were genetically linked pancreatitis (21.10%), pancreatic divisum (11.60%), alcohol-induced CP (11.00%), and biliary tract disease (6.90%). Six percent of patients were insulin-dependent before TPIAT. Among pediatric patients, the majority of TPIATs were performed on patients with genetically linked CP (74.40%). Twenty-four percent of pediatric patients had idiopathic CP, and one pediatric patient had pancreatic divisum (0.44%).
Insulin independence and insulin requirements after TPIAT generally appear to correlate with higher islet yield, defined as the number of islet equivalents (IEs) transplanted per kilogram (kg) of recipient body weight[16,35,36,42,45,46]. However, overall TPIAT outcomes are likely multifactorial[31]. Several studies, including White et al[31], suggest additional factors may influence whether a patient achieves insulin independence after TPIAT: Prior pancreatic operations; poor islet yield due to pathogenic severity, calcification, and/or fibrosis; pathologic damage preventing islet purification of pancreatic tissue; toxic damage from reagents with high levels of endotoxins used in islet purification; the intraportal site being a suboptimal place for islet transplantation as it does not regulate insulin or glucose secretion; and chronic rejection of islet allotransplants[31,35].
Wang et al[36] showed that prior surgery is strongly correlated with pancreatic fibrosis and islet yield. Fewer islets are obtained from more fibrotic pancreases, because of both the disease process itself and increased difficulty in islet processing for transplant[36]. Prior history of pancreatic surgery may be used to predict postoperative islet function and determine the optimal timing for TPIAT surgery[36]. Sutton et al[44] make a similar observation regarding TPIAT in pediatric patients with genetically linked CP. Sixty-three percent of patients in Sutton et al[44] have the CFTR mutation. The authors advocate against trial resections or decompression surgeries before TP, as this treatment often compromises future endocrine function by limiting islet yield. None of the patients who had undergone previous pancreatic operations were insulin independent after TPIAT, and patients with previous pancreatic operations had approximately half the islet yield compared to patients without previous surgery.
The probability of TPIAT success is predicted by the morphologic features of the pancreas[42]. With plain films, ultrasonography, computed tomography (CT), or Endoscopic retrograde cholangiopancreatography (ERCP), Wahoff et al[42] suggest that the pre-operative prediction of the severity of the fibrosis helps estimate the number of islets available for autotransplantation. The severity of pain is notably an unreliable predictor of pancreas morphology and islets available for transplantation[42]. ERCP with transduodenal biopsy allows for assessing pancreas morphology directly and is likely the most useful means of evaluating islets pre-operatively[42].
Multiple papers suggest that women have better C-peptide positivity and glycemic control postoperatively because they often receive more IEs/kg. Univariate analyses by Ahmad et al[29] demonstrate that female gender, lower body weight, lower mean insulin requirements for the first 24 h postoperatively, and lower mean insulin requirements at the time of discharge are also associated with insulin independence. Seventeen of 18 insulin-free patients were female in Ahmad et al[29]. Multiple logistic regressions including gender, body mass index (BMI), and IEs/kg found gender to be an important independent variable. In their series, men were heavier than women on average by 10 kg, and they explained these findings as the result of weight differences among the sexes, saying patients with increased BMI are less likely to benefit from TPIAT and ought to be counseled on losing weight before surgery as their likelihood of glycemic control afterward is associated with their BMI[29].
Insulin independence and insulin requirements after TPIAT appear to correlate with higher islet yield in pediatric patients as well[17,44]. Multivariate analysis by Chinnakotla et al[17], demonstrated male gender, lower body surface area, and higher total IEs/kg were associated with insulin independence after TPIAT in pediatric populations. Total IEs greater than 2500 IE/kg was the most strongly associated with insulin independence.
In addition to gender, BMI, and previous pancreatic operations, the amount of time between CP diagnosis and TPIAT procedure has been demonstrated to have a direct impact on islet yield[33]. Gruessner et al[33] discovered that outcomes improved when patients were referred at earlier disease stages, before surgical procedures, and after inadequate endoscopies. Gruessner et al[33] was the first paper to document fully robotically assisted TPIAT. They found that approximately 80% of their patients had undergone previous surgical procedures and that 91% had abnormal results on preoperative continuous glucose monitoring tests[33].
The auto-transplanted islet function appears to be durable[15,47]. Wilson et al[15] conducted one of the largest series reviewing long-term outcomes after TPIAT. The study found that insulin independence rates decline over time but that most patients maintain stable glycemic control past 13 years post-operation and have minimal long-term complications associated with DM. Wilson et al[15] hypothesize that the toxic environment created by the liver is what ultimately contributes to declines in islet function over time.
While preservation of beta cell function is an important consideration, the success of TPIAT is ultimately determined by its ability to relieve pain and restore QoL in patients with CP[16]. Constant pain is the strongest predictor of poor QoL in patients with CP[48]. Relieving pain and reducing narcotic use is the primary objective of TPIAT[49]. In our meta-analysis, narcotic independence was achieved in 53.5% of adults post-TPIAT and 51.9% of children post-TPIAT[15-17,25,29,30,32-35,37,39,43,44,50-52].
Some authors suggest that CP patients often have multiple comorbidities that cause pain after TP[53]. Patients with these additional comorbidities often require opioid analgesia beyond patients undergoing TP without comorbidities[53]. Additionally, long-term use of opioids can lead to dependence and addiction, causing long-term analgesic requirements[33,53].
Surgical intervention earlier in the course of the disease is associated with improved pain control and less narcotic use[26,54,55]. Interestingly, Bellin et al[18] demonstrated that TPIAT benefits even those without evident CP by improving QoL and reducing narcotic use. Patients with recurrent acute pancreatitis and limited surgical treatment options after medical and endoscopic therapy failed to remit their pain had outcomes similar to those patients with CP[18].
Several studies, including Colling et al[35], demonstrated that TPIAT can be an effective and safe treatment option for patients with cystic fibrosis (CF) and debilitating CP. Of note, these patients are likely at increased risk for pulmonary and luminal GI tract complications. Colling et al[35] had a cohort of 20 patients with CF and 19 CFTR carriers with TPIAT outcomes similar to other patient populations. Sutton et al[44] also demonstrated TPIAT was a successful treatment option in patients with genetically linked pancreatitis, finding narcotic independence rates of 63% and drastic decreases in narcotic requirements.
Fan et al[34] demonstrated that laparoscopic TPIAT (L-TPIAT) can be beneficial to CP patients as it reduced total operative and islet isolation time, shortened length of stay, and minimized the surgical pain spike compared to open and robot-assisted TPIATs. Fan et al[34] suggest reducing these metrics by performing L-TPIATs allows for opioid independence to be achieved more quickly. To Fan et al[34]’s point, Wilson et al[15] argue that minimizing warm ischemia time to the islets is one of the most important considerations during the operation.
While our meta-analysis spanned 37 years, data on TPIAT outcomes remains sparse. Various researchers have used a variety of evaluative tools to evaluate pain after TPIAT, including visual analog pain scores and inference scores. Treatment centers have followed patients for various lengths of time post-operatively, tracking their insulin independence at a variety of different post-operative times. Our meta-analysis draws on a large cohort of patients with CP undergoing TPIAT. The current study incorporates multiple treatment centers in two countries and a diversity of disease etiology, duration, and severity. We chose to evaluate the most objective data regarding post-operative pain and endocrine function: insulin and narcotic independence. As such, our meta-analysis uses the strengths of the available literature to maximize the reliability of our results.
TPIAT produces acceptable levels of pain relief in patients with CP. Over half of patients were narcotic-independent post-operatively. Regaining endocrine function after TPIAT appears to be multifactorial as a majority of patients continue to remain insulin-dependent following surgery, albeit there is a substantial improvement in glycemic control as reflected by lower HBA1C levels in the postoperative period. Future studies should evaluate the discussed measures before and after surgery for comparison. Clear definitions of patient populations, surgical procedures as well as post-surgical care are needed to limit heterogeneity in outcomes. Long-term prospective studies will be needed to further examine the longevity of insulin and opioid independence.
Debilitating abdominal pain and diabetes mellitus are hallmark clinical manifestations of chronic pancreatitis (CP). Current management strategies revolve around pain mitigation and treatment of endocrine failure. One available treatment option is total pancreatectomy with islet cell auto tran
Emerging data from multiple studies highlight that TP-IAT results in considerable pain relief and insulin independence; however, systemic evidence from high-quality studies is limited.
We performed a systemic review and meta-analysis to evaluate clinical outcomes such as pain control and glucose intolerance following TP-IAT.
A comprehensive literature search spanning Pubmed, EMBASE, and Cochrane databases was performed from inception to March 2019. Studies conducted on outcomes of TP-IAT in patients with CP were identified. Comprehensive meta-analysis software was used to extract and analyze data. The random-effects model was used for all variables. Heterogeneity was assessed using the I2 measure and Cochrane Q-statistic. Publication bias was assessed using Egger’s test.
Our meta-analysis evaluated a total of 1100 patients across 21 studies. We found that TI-IAT results in narcotic independence in over 50% of adult and pediatric patients with CP. IAT results in meaningful islet cell function with insulin independence noted in almost one-third of adults and nearly half of pediatric patients following surgery.
TP-IAT results in acceptable narcotic independence and preservation of beta cell function.
Long-term prospective studies with clear definitions of patient populations, surgical procedures, and post-surgical care are needed to definitively evaluate insulin and narcotic independence before and after surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujino Y, Japan; Nah YW, South Korea S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Lankisch PG, Löhr-Happe A, Otto J, Creutzfeldt W. Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54:148-155. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820-828. [PubMed] [Cited in This Article: ] |
| 3. | Olesen SS, Büyükuslu A, Køhler M, Rasmussen HH, Drewes AM. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology. 2019;19:245-251. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Malka D, Hammel P, Sauvanet A, Rufat P, O'Toole D, Bardet P, Belghiti J, Bernades P, Ruszniewski P, Lévy P. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology. 2000;119:1324-1332. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M; HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5:153-199. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Drewes AM, Bouwense SAW, Campbell CM, Ceyhan GO, Delhaye M, Demir IE, Garg PK, van Goor H, Halloran C, Isaji S, Neoptolemos JP, Olesen SS, Palermo T, Pasricha PJ, Sheel A, Shimosegawa T, Szigethy E, Whitcomb DC, Yadav D; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17:720-731. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: A treatment algorithm. Best Pract Res Clin Gastroenterol. 2010;24:323-335. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Issa Y, van Santvoort HC, van Goor H, Cahen DL, Bruno MJ, Boermeester MA. Surgical and endoscopic treatment of pain in chronic pancreatitis: a multidisciplinary update. Dig Surg. 2013;30:35-50. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Olesen SS, Bouwense SA, Wilder-Smith OH, van Goor H, Drewes AM. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology. 2011;141:536-543. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39-47. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Delmonico FL. Analyzing risk factors of renal transplant outcome. Transplantation. 2000;69:1-2. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Kirchner VA, Dunn TB, Beilman GJ, Chinnakotla S, Pruett TL, Wilhelm JJ, Schwarzenberg SJ, Freeman ML, Bellin MD. Total Pancreatectomy With Islet Autotransplantation for Acute Recurrent and Chronic Pancreatitis. Curr Treat Options Gastroenterol. 2017;15:548-561. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Morgan K, Owczarski SM, Borckardt J, Madan A, Nishimura M, Adams DB. Pain control and quality of life after pancreatectomy with islet autotransplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16:129-33; discussion 133. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Chinnakotla S, Radosevich DM, Dunn TB, Bellin MD, Freeman ML, Schwarzenberg SJ, Balamurugan AN, Wilhelm J, Bland B, Vickers SM, Beilman GJ, Sutherland DE, Pruett TL. Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg. 2014;218:530-543. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Wilson GC, Sutton JM, Abbott DE, Smith MT, Lowy AM, Matthews JB, Rilo HL, Schmulewitz N, Salehi M, Choe K, Brunner J, Hanseman DJ, Sussman JJ, Edwards MJ, Ahmad SA. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg. 2014;260:659-65; discussion 665. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, Chinnakotla S, Vickers SM, Bland B, Balamurugan AN, Freeman ML, Pruett TL. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409-24; discussion 424. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Chinnakotla S, Bellin MD, Schwarzenberg SJ, Radosevich DM, Cook M, Dunn TB, Beilman GJ, Freeman ML, Balamurugan AN, Wilhelm J, Bland B, Jimenez-Vega JM, Hering BJ, Vickers SM, Pruett TL, Sutherland DE. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis: indication, surgical techniques, postoperative management, and long-term outcomes. Ann Surg. 2014;260:56-64. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Bellin MD, Kerdsirichairat T, Beilman GJ, Dunn TB, Chinnakotla S, Pruett TL, Radosevich DR, Schwarzenberg SJ, Sutherland DE, Arain MA, Freeman ML. Total Pancreatectomy With Islet Autotransplantation Improves Quality of Life in Patients With Refractory Recurrent Acute Pancreatitis. Clin Gastroenterol Hepatol. 2016;14:1317-1323. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2012. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Cited in This Article: ] |
| 20. | Alper BS, Haynes RB. Reply to 'Shaneyfelt T. Pyramids are guides not rules: the evolution of the evidence pyramid. Evid Based Med 2016;21:121-2'. Evid Based Med. 2016;21:200. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58:365-382. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Garcae G, Pollard CA, Illouz S, Webb M, Metcalfe MS, Dennison AR. Patient satisfaction and cost-effectiveness following total pancreatectomy with islet cell transplantation for chronic pancreatitis. Pancreas. 2013;42:322-328. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Solomina J, Gołębiewska J, Kijek MR, Kotukhov A, Bachul PJ, Basto L, Gołąb K, Konsur E, Cieply K, Fillman N, Wang LJ, Thomas CC, Philipson LH, Tibudan M, Dębska-Ślizień A, Fung J, Gelrud A, Matthews JB, Witkowski P. Pain Control, Glucose Control, and Quality of Life in Patients With Chronic Pancreatitis After Total Pancreatectomy With Islet Autotransplantation: A Preliminary Report. Transplant Proc. 2017;49:2333-2339. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Yang CJ, Bliss LA, Freedman SD, Sheth S, Vollmer CM, Ng SC, Callery MP, Tseng JF. Surgery for chronic pancreatitis: the role of early surgery in pain management. Pancreas. 2015;44:819-823. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Chinnakotla S, Beilman GJ, Dunn TB, Bellin MD, Freeman ML, Radosevich DM, Arain M, Amateau SK, Mallery JS, Schwarzenberg SJ, Clavel A, Wilhelm J, Robertson RP, Berry L, Cook M, Hering BJ, Sutherland DE, Pruett TL. Factors Predicting Outcomes After a Total Pancreatectomy and Islet Autotransplantation Lessons Learned From Over 500 Cases. Ann Surg. 2015;262:610-622. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Fazlalizadeh R, Moghadamyeghaneh Z, Demirjian AN, Imagawa DK, Foster CE, Lakey JR, Stamos MJ, Ichii H. Total pancreatectomy and islet autotransplantation: A decade nationwide analysis. World J Transplant. 2016;6:233-238. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Ahmad SA, Lowy AM, Wray CJ, D'Alessio D, Choe KA, James LE, Gelrud A, Matthews JB, Rilo HL. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680-687. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Najarian JS, Sutherland DE, Baumgartner D, Burke B, Rynasiewicz JJ, Matas AJ, Goetz FC. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg. 1980;192:526-542. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | White SA, Davies JE, Pollard C, Swift SM, Clayton HA, Sutton CD, Weymss-Holden S, Musto PP, Berry DP, Dennison AR. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg. 2001;233:423-431. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Mokadem M, Noureddine L, Howard T, McHenry L, Sherman S, Fogel EL, Watkins JL, Lehman GA. Total pancreatectomy with islet cell transplantation vs intrathecal narcotic pump infusion for pain control in chronic pancreatitis. World J Gastroenterol. 2016;22:4160-4167. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Gruessner RW, Cercone R, Galvani C, Rana A, Porubsky M, Gruessner AC, Rilo H. Results of open and robot-assisted pancreatectomies with autologous islet transplantations: treating chronic pancreatitis and preventing surgically induced diabetes. Transplant Proc. 2014;46:1978-1979. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Fan CJ, Hirose K, Walsh CM, Quartuccio M, Desai NM, Singh VK, Kalyani RR, Warren DS, Sun Z, Hanna MN, Makary MA. Laparoscopic Total Pancreatectomy With Islet Autotransplantation and Intraoperative Islet Separation as a Treatment for Patients With Chronic Pancreatitis. JAMA Surg. 2017;152:550-556. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Colling KP, Bellin MD, Schwarzenberg SJ, Berry L, Wilhelm JJ, Dunn T, Pruett TL, Sutherland DER, Chinnakotla S, Dunitz JM, Beilman GJ. Total Pancreatectomy With Intraportal Islet Autotransplantation as a Treatment of Chronic Pancreatitis in Patients With CFTR Mutations. Pancreas. 2018;47:238-244. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Wang H, Desai KD, Dong H, Owzarski S, Romagnuolo J, Morgan KA, Adams DB. Prior surgery determines islet yield and insulin requirement in patients with chronic pancreatitis. Transplantation. 2013;95:1051-1057. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Rabkin JM, Olyaei AJ, Orloff SL, Geisler SM, Wahoff DC, Hering BJ, Sutherland DE. Distant processing of pancreas islets for autotransplantation following total pancreatectomy. Am J Surg. 1999;177:423-427. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Rossi RL, Soeldner JS, Braasch JW, Heiss FW, Shea JA, Nugent FW, Watkins E Jr, Silverman ML, Bolton J. Segmental pancreatic autotransplantation with pancreatic ductal occlusion after near total or total pancreatic resection for chronic pancreatitis. Results at 5- to 54-month follow-up evaluation. Ann Surg. 1986;203:626-636. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Argo JL, Contreras JL, Wesley MM, Christein JD. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg. 2008;74:530-6; discussion 536. [PubMed] [Cited in This Article: ] |
| 40. | Toledo-Pereyra LH. Islet cell autotransplantation after subtotal pancreatectomy. Arch Surg. 1983;118:851-858. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Valente U, Arcuri S, Barocci S. Islet and segmental pancreatic autotransplantation after pancreatectomy: follow up of 25 patients for up to five years. Transplant Proc. 1985;363-365. [Cited in This Article: ] |
| 42. | Wahoff DC, Paplois BE, Najarian JS, Farney AC, Leonard AS, Kendall DM, Roberston RR, Sutherland DE. Islet Autotransplantation after total pancreatectomy in a child. J Pediatr Surg. 1996;31:132-5; discussion 135. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Bellin MD, Forlenza GP, Majumder K, Berger M, Freeman ML, Beilman GJ, Dunn TB, Pruett TL, Murati M, Wilhelm JJ, Cook M, Sutherland DE, Schwarzenberg SJ, Chinnakotla S. Total Pancreatectomy With Islet Autotransplantation Resolves Pain in Young Children With Severe Chronic Pancreatitis. J Pediatr Gastroenterol Nutr. 2017;64:440-445. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Sutton JM, Schmulewitz N, Sussman JJ, Smith M, Kurland JE, Brunner JE, Salehi M, Choe KA, Ahmad SA. Total pancreatectomy and islet cell autotransplantation as a means of treating patients with genetically linked pancreatitis. Surgery. 2010;148:676-85; discussion 685. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Bellin MD, Freeman ML, Schwarzenberg SJ, Dunn TB, Beilman GJ, Vickers SM, Chinnakotla S, Balamurugan AN, Hering BJ, Radosevich DM, Moran A, Sutherland DE. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:793-799. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Rodriguez Rilo HL, Ahmad SA, D'Alessio D, Iwanaga Y, Kim J, Choe KA, Moulton JS, Martin J, Pennington LJ, Soldano DA, Biliter J, Martin SP, Ulrich CD, Somogyi L, Welge J, Matthews JB, Lowy AM. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978-989. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Robertson RP, Lanz KJ, Sutherland DE, Kendall DM. Prevention of diabetes for up to 13 years by autoislet transplantation after pancreatectomy for chronic pancreatitis. Diabetes. 2001;50:47-50. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Mullady DK, Yadav D, Amann ST, O'Connell MR, Barmada MM, Elta GH, Scheiman JM, Wamsteker EJ, Chey WD, Korneffel ML, Weinman BM, Slivka A, Sherman S, Hawes RH, Brand RE, Burton FR, Lewis MD, Gardner TB, Gelrud A, DiSario J, Baillie J, Banks PA, Whitcomb DC, Anderson MA; NAPS2 Consortium. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77-84. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Shindo Y, Kanak MA. Total pancreatectomy with islet autotransplantation: recent updates and outcomes. Curr Opin Organ Transplant. 2017;22:444-451. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Wahoff DC, Papalois BE, Najarian JS, Kendall DM, Farney AC, Leone JP, Jessurun J, Dunn DL, Robertson RP, Sutherland DE. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg. 1995;222:562-75; discussion 575. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Garcea G, Weaver J, Phillips J, Pollard CA, Ilouz SC, Webb MA, Berry DP, Dennison AR. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: a series of 85 consecutive patients. Pancreas. 2009;38:1-7. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Bellin MD, Sutherland DE. Pediatric islet autotransplantation: indication, technique, and outcome. Curr Diab Rep. 2010;10:326-331. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Ong SL, Gravante G, Pollard CA, Webb MA, Illouz S, Dennison AR. Total pancreatectomy with islet autotransplantation: an overview. HPB (Oxford). 2009;11:613-621. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | van der Gaag NA, van Gulik TM, Busch OR, Sprangers MA, Bruno MJ, Zevenbergen C, Gouma DJ, Boermeester MA. Functional and medical outcomes after tailored surgery for pain due to chronic pancreatitis. Ann Surg. 2012;255:763-770. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Ahmed Ali U, Nieuwenhuijs VB, van Eijck CH, Gooszen HG, van Dam RM, Busch OR, Dijkgraaf MG, Mauritz FA, Jens S, Mast J, van Goor H, Boermeester MA; Dutch Pancreatitis Study Group. Clinical outcome in relation to timing of surgery in chronic pancreatitis: a nomogram to predict pain relief. Arch Surg. 2012;147:925-932. [PubMed] [DOI] [Cited in This Article: ] |