Published online Mar 22, 2015. doi: 10.5498/wjp.v5.i1.154
Peer-review started: October 1, 2014
First decision: November 27, 2014
Revised: January 8, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: March 22, 2015
AIM: To systematically review studies measuring peripheric brain-derived neurotrophic factor (BDNF) levels on first-episode psychosis patients and variables related to them.
METHODS: A systematic search was made of articles published in the Medline database from 2002 up to June 2014. Included are original studies that report enzyme-linked immunosorbent assay measurement of BDNF levels in serum or plasma in patients with a diagnosis of first episode psychosis (FEP) and age- and gender- matched healthy controls.
RESULTS: Of the initially identified 147 articles, only 18 satisfied the inclusion criteria. Of this, 15 found a significant reduction in patients with FEP compared with age- and gender - matched controls.
CONCLUSION: Peripheral BDNF levels are generally reduced in FEP patients. There are some factors that may influence BDNF levels that need to be further studied. Furthermore, a future meta-analysis in this topic is needed.
Core tip: Brain-derived neurotrophic factor (BDNF) has an important role during brain development and various studies have reported altered peripheral BDNF levels in schizophrenia, but findings are inconsistent. Some studies have been carried out specifically in first episode patients to address this issue. In the present study we have systematically reviewed studies measuring BDNF levels in first episode psychosis (FEP) patients compared to healthy controls and variables related to them. Most studies report reduced BDNF levels in FEP patients but some factors that may influence BDNF levels need to be further studied.
- Citation: Toll A, Mané A. Brain-derived neurotrophic factor levels in first episode of psychosis: A systematic review. World J Psychiatr 2015; 5(1): 154-159
- URL: https://www.wjgnet.com/2220-3206/full/v5/i1/154.htm
- DOI: https://dx.doi.org/10.5498/wjp.v5.i1.154
Brain-derived neurotrophic factor (BDNF) is the most widely distributed neurotrophin in the central nervous system and is highly expressed in the hippocampus and the prefrontal cortex (areas implicated in schizophrenia symptoms[1]. BDNF has an important role during brain development as it is implicated in many essential functions, including neurogenesis, neuronal differentiation, survival and normal maturation of neurodevelopmental pathways[2]. BDNF also has a significant role in the adult brain, regulating neuronal integrity, promoting synaptic plasticity, modulating synthesis, metabolism and release of neurotransmitters (dopamine, γ-aminobutyric acid, serotonin and glutamate), and moderating neuroplasticity processes[3].
As abnormalities in brain development and plasticity have been related to the pathophysiology of schizophrenia, there is a growing interest in understanding the role of BDNF in this disease. Interestingly, BDNF crosses the blood-brain barrier and serum concentrations strongly correlate with brain levels[4]. Low serum BDNF levels have been associated with decreased hippocampal volume, an area that has been strongly related to schizophrenia[5]. A meta-analysis conducted by Green et al[6] in 2011 indicated that BDNF levels were altered in patients with schizophrenia, with numerous studies, but not all, reporting reduced levels. They concluded that peripheral BDNF levels were moderately reduced in schizophrenia samples, including drug naïve and medicated patients, when compared with age-matched healthy controls. They also found an accelerated decrease with age, although they observed a high heterogeneity in BDNF levels between the different studies. Furthermore, their results could not support the greater decrease in men than in women that had previously been observed. The reasons for the heterogeneity in BDNF levels between different studies could be due in part by the fact that some patients were evaluated while on antipsychotic treatment. Atypical antipsychotics may increase, whereas treatment with conventional antipsychotics may decrease, peripheral BDNF levels[7]. Moreover, they did not take into account illness characteristics, such as illness stage. BDNF alterations throughout the course of illness are still unclear.
Identifying BDNF alterations in first episode psychosis (FEP) is a preliminary step to understanding the role of BDNF in schizophrenia pathophysiology, as first episode patients are not affected by medication effects (in some cases), or other factors related with chronicity[8]. Several studies have been carried out in FEP patients to identify peripheral BDNF alterations. Therefore, the aim of the present study was to systematically review these studies measuring peripheral BDNF levels in FEP (drug-naïve or medicated) patients and analyze the variables related with them.
A systematic search was made of articles published in the Medline database using the following keywords: “schizophrenia”, “psychosis”, and “first episode” in combination with “bdnf”, “serum”, “plasma”, and “peripheral”. Articles in both English and Spanish published from 2002 up to June 2014 were selected. Indexed references in the retrieved articles that met the inclusion criteria were manually searched for additional relevant studies.
Included in the systematic review are original studies that report enzyme-linked immunosorbent assay measurement of BDNF levels in serum or plasma[6] in patients with a diagnosis of FEP and age- and gender-matched healthy controls. We excluded studies reporting data for patients with comorbid neurologic disorders or other psychiatric illnesses. We also excluded studies that reported peripheral BDNF levels in chronic schizophrenia patients and studies that only reported BDNF genetic data.
From the included studies, we assessed age and gender characteristics. We analyzed statistical differences in age and gender percentage between studies with lower BDNF levels than controls and those that did not with a t-test.
A statistical review has been performed by a biomedical statistician.
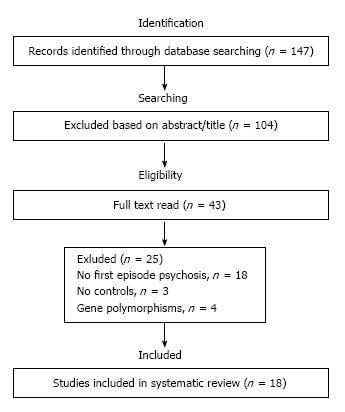
A diagram describing the selection of studies is presented in Figure 1. Of the initially identified 147 articles, only 18 satisfied the inclusion criteria[5,9-25]. Characteristics of these studies are described in Table 1. Seven articles reported BDNF levels in plasma and 11 studies reported levels in blood serum. Moreover, 13 articles reported BDNF levels only in drug-naïve patients and 5 studies reported BDNF levels in medicated patients (the most used antipsychotics were olanzapine, risperidone, quetiapine, aripiprazole and clozapine). When a study measured blood BDNF levels at two different time points, such as the studies that reported BDNF levels in schizophrenia patients before and after antipsychotic treatments, we included only data from the baseline without treatment. A detailed description of the results from the included studies is summarized in Table 2.
| Ref. | Sample | Age (yr) | Matched for | Illness duration (yr) | Medication | BDNF measurement |
| Jockers-Scherübl et al[9] | 157 FEP | 31.8 | Age, gender | Not reported | Drug naïve | Serum |
| 72 Ctl | 32.3 | |||||
| Zakharyan et al[10] | 25 FEP | 25.3 ± 9.2 | Age, gender | 18.4 ± 7.2 | Drug naïve | Plasma |
| 105 Ctl | 37.3 ± 11.3 | |||||
| Murphy et al[11] | 15 FEP | 18.6 ± 3.3 | Age, gender | Not reported | Quetiapine | Serum |
| 15 Ctl | 18.5 ± 3.1 | |||||
| Sotiropoulou et al[12] | 50 FEP | 29.84 ± 8.20 | Age, gender | Not reported | Drug naïve | Serum |
| 50 Ctl | 31.36 ± 7.96 | |||||
| Ruiz de Azua et al[13] | 47 FEP | 24.3 ± 8.5 | Age, gender, education level | Not reported | Drug naïve, Rsp, Olz | Plasma |
| 47 Ctl | 24.0 ± 8.8 | |||||
| Yoshimura et al[14] | 50 FEP | 30.8 ± 5.3 | Age, gender | Not reported | Apz | Plasma |
| 50 Ctl | 32.3 ± 7.1 | |||||
| Rizos et al[5] | 20 FEP | 30.8 ± 10.5 | Age, gender, education level, employment | Not reported | Drug naïve | Serum |
| 21 Ctl | 34.0 ± 4.7 | |||||
| González-Pinto et al[15] | 18 FEP | 24.39 ± 6.53 | Age, gender | Not reported | Drug naïve1 | Plasma |
| 18 Ctl | 25.19 ± 5.95 | |||||
| Rizos et al[16] | 37 FEP | 26.81 ± 9.22 | Age, gender | Not reported | Drug naïve | Serum |
| 22 Ctl | 26.59 ± 4.47 | |||||
| Jindal et al[17] | 41 FEP | 22.40 ± 5.47 | Age, gender | Not reported | Drug naïve | Serum |
| 41 Ctl | 22.31 ± 5.67 | |||||
| Goto et al[18] | 18 FEP | 29 ± 11 | Age, gender | 9.4 ± 6.8 | Drug naïve, Rsp/Olz/Apz | Serum |
| 18 Ctl | 30 ± 11 | |||||
| Pillai et al[19] | 34 FEP | 32.19 ± 8.74 | Age, gender | Not reported | Drug naïve | Plasma |
| 36 Ctl | 38.30 ± 1.26 | |||||
| Rizos et al[20] | 31 FEP | 26.81 ± 9.22 | Age, gender, education level, employment | Not reported | Drug naïve | Serum |
| 22 Ctl | 26.81 ± 9.22 | |||||
| Chen da et al[21] | 88 FEP | 29.2 ± 9.6 | Age, gender, education level, smoking | 23.4 ± 19.1 | Drug naïve | Serum |
| 90 Ctl | 29.8 ± 9.8 | |||||
| Rizos et al[22] | 14 FEP | 25.4 ± 5.8 | Age, gender, education level, employment | Not reported | Drug naïve | Serum |
| 15 Ctl | 26.6 ± 5.0 | |||||
| Buckley et al[23] | 15 FEP | 21.00 ± 8.83 | Age, gender | 25.2 ± 33.0 | Drug naïve | Plasma |
| 14 Ctl | 25.00 ± 5.72 | |||||
| Palomino et al[24] | 48 FEP | 23.7 ± 1.0 | Age, gender | Not reported | Atypical, mood stabilizers | Plasma |
| 43 Ctl | 25.5 ± 0.8 | |||||
| Pirildar et al[25] | 22 FEP | 27.81 ± 9.54 | Age, gender | 15.2 ± 13.0 | Drug naïve1 | Serum |
| 22 Ctl | 25.7 ± 5.8 |
| Ref. | FEP (ng/mL) | Controls (ng/mL) | Results | Effect size d (CI) |
| Jockers-Scherübl et al[9] | 13.1 ± 5.9 | 13.2 ± 0.2 | No difference | 0.10 (-0.18-0.38) |
| Zakharyan et al[10] | 0.176 ± 0.014 | 0.24 ± 0.3 | ↓ in FEP | 1.21 (0.75-1.67) |
| Murphy et al[11] | Missing data | Missing data | No difference | - |
| Sotiropoulou et al[12] | 12620 ± 1860 | 14520 ± 2180 | ↓ in FEP | 0.64 (0.23-1.04) |
| Ruiz de Azua et al[13] | 6.09 ± 3.70 | 9.19 ± 4.21 | ↓ in FEP | 0.66 (0.24-1.07) |
| Yoshimura et al[14] | 0.7 ± 0.4 | Missing data | ↓ in FEP | 0.43 (0.03-0.82) |
| Rizos et al[5] | 9.76 ± 4.61 | 15.33 ± 6.34 | ↓ in FEP | 0.99 (0.34-1.64) |
| González-Pinto et al[15] | 4.09 ± 2.31 | 5.80 ± 2.66 | ↓ in FEP | - |
| Rizos et al[16] | 18.87 ± 8.23 | 29.20 ± 7.73 | ↓ in FEP | 0.72 (0.17-1.26) |
| Jindal et al[17] | 0.09 ± 0.03 | 0.117 ± 0.04 | ↓ in FEP | 0.54 (0.10-0.99) |
| Goto et al[18] | Missing data | Missing data | No difference | 0.38 (-0.28-1.04) |
| Pillai et al[19] | Missing data | Missing data | ↓ in FEP | 0.53 (0.05-1.00) |
| Rizos et al[20] | Missing data | Missing data | ↓ in FEP | - |
| Chen da et al[21] | 9.0 ± 4.2 | 12.1 ± 2.2 | ↓ in FEP | 0.92 (0.61-1.23) |
| Rizos et al[22] | 23.90 ± 5.99 | 30.00 ± 8.43 | ↓ in FEP | 0.83 (0.07-1.59) |
| Buckley et al[23] | 0.017 ± 0.003 | 0.049 ± 0.007 | ↓ in FEP | 1.37 (0.56-2.18) |
| Palomino et al[24] | 4.19 ± 2.26 | 7.55 ± 4.31 | ↓ in FEP | 0.56 (0.13-0.97) |
| Pirildar et al[25] | 14.2 ± 8.1 | 26.8 ± 9.3 | ↓ in FEP | 1.07 (0.93-1.7) |
Of the 18 articles that measured BDNF levels at baseline, 15 found a significant reduction in patients with FEP compared with age- and gender-matched controls.
There were no statistical differences in mean age between those studies that found lower BDNF levels in patients than those that did not (26.18 ± 3.89 vs 29.11 ± 3.50, P = 0.24) The percentage of men was lower in the studies that found decreased BDNF levels in patients compared to those that did not (47.89% vs 54.71%, P = 0.36 ).
We found 15 out of 18 articles found a reduction in BDNF levels compared to age- and gender-matched controls. It is important to note that 13/18 studies included only drug-naïve patients and 2 included drug-naïve and medicated patients. Televe out of 15 studies that found a decrease were conducted in drug-naïve patients whereas two of the three studies that found no alteration in BDNF included medicated patients. These results are consistent with some studies pointing out normalization in BDNF levels with treatment. However, other additional factors may influence BDNF alterations.
Another factor that could affect BDNF levels is the mean age of patients, as a greater reduction in peripheral BDNF in schizophrenia was reported with increasing age in Green et al[6]. We did not find statistical differences in mean age between those studies that found lower BDNF levels in patients than those that did not. However we must take into account that our age variance was much smaller than in Green et al[6] study.
On the other hand, although some articles have described a greater reduction in BDNF in males than females with schizophrenia[6], this does not appear to be a factor in the present analysis, even after taking into account the size effect (results upon demand). This results are in agreement with Green et al[6] who found no a reduction in both men and women in BDNF after excluding an outlier.
Patients clinical characteristics, could also affect BDNF levels. It would be interesting to conduct a prospective study on high risk psychosis patients through different illness stages to clarify this issue.
Another important variable to consider is substance use, particularly cannabis use. Cannabis use is very common in first episode patients, with a prevalence ranging from 30% to 60%[26]. Moreover, in non-psychotic patients, it has been described that acute cannabis use can initially increase, whereas chronic use can decrease, peripheral BDNF levels[27]. However, cannabis use was an exclusion criterion in most studies, but not in all. Specifically, the article by Jockers-Scherübl at al[9] compared BDNF levels between FEP patients and controls that used and did not use cannabis[9]. They found increased BDNF levels in FEP cannabis-users than non-users and controls. These results are in disagreement with a more recent study[27]. Further research should therefore be performed to clarify the effects of cannabis use on BDNF levels in FEP patients. The effect of the val66met BDNF polymorphism and the interaction between this polymorphism and cannabis use could also affect BDNF levels. A Val to Met substitution at codon 66 of the BDNF gene, is known to result in less efficient intracellular trafficking and decreased activity-dependent BDNF secretion and has been related to psychosis emergence[28]. Furthermore a study has described an interaction effect between cannabis, this polymorphism and gender, on the risk of psychosis emergence[29].
Other environment factor should also affect BDNF levels. A history of childhood trauma has been related to a reduction of BDNF levels[30].
A limitation of this type of systematic review is that some relevant studies, including those that are unpublished, may be overlooked. Another limitation is the marked heterogeneity of the articles included in the review in relation to the sample size, the mean age of the patients, the proportion of men/women, the illness duration (only reported in six studies), the medication type (in patients receiving treatment), and the type of symptomatology patients were presenting. Furthermore, we did not take into account the effect of the BDNF polymorphism, and it could have multiplicative on lower BDNF levels.
Peripheral BDNF levels are generally reduced in FEP patients. However, there are some factors that may influence BDNF levels that need to be further studied, which will help to resolve inconsistencies in the literature. Furthermore, a future meta-analysis on this topic would help to clarify BDNF alterations in FEP.
Altered brain-derived neurotrophic factor (BDNF) levels have been described in schizophrenia, and have been related to brain abnormalities, but findings are still inconsistent. Moreover, BDNF alterations throughout the illness course are still unclear.
Neuroimaging in schizophrenia; neurodevelopment in schizophrenia; neuroplasticity in schizophrenia; neurodegeneration in schizophrenia.
This is the first work that systematically reviews studies measuring peripheric BDNF levels in first episode patients, a group of patients not affected by cronicity.
Comprehending BDNF alterations in first episode patients is a preliminary step to understanding the role of BDNF in schizophrenia pathophysiology and brain structure alterations.
Neurotrophic factors are a family of proteins that are responsible for the growth and survival of developing neurons and the maintenance of mature neurons.
This is an interesting manuscript.
P- Reviewer: Forero DA, Maziade M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919-2937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 771] [Cited by in F6Publishing: 789] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 2. | Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099-10102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 675] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 3. | Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: findings in schizophrenia. Curr Opin Psychiatry. 2011;24:122-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, Danker-Hopfe H, Gass P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Rizos EN, Papathanasiou M, Michalopoulou PG, Mazioti A, Douzenis A, Kastania A, Nikolaidou P, Laskos E, Vasilopoulou K, Lykouras L. Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr Res. 2011;129:201-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 7. | Favalli G, Li J, Belmonte-de-Abreu P, Wong AH, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Buckley PE, Evans D. First-episode schizophrenia. A window of opportunity for optimizing care and outcomes. Postgrad Med. 2006;Spec No:5-19. [PubMed] [Cited in This Article: ] |
| 9. | Jockers-Scherübl MC, Danker-Hopfe H, Mahlberg R, Selig F, Rentzsch J, Schürer F, Lang UE, Hellweg R. Brain-derived neurotrophic factor serum concentrations are increased in drug-naive schizophrenic patients with chronic cannabis abuse and multiple substance abuse. Neurosci Lett. 2004;371:79-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Zakharyan R, Boyajyan A. Brain-derived neurotrophic factor blood levels are decreased in schizophrenia patients and associate with rs6265 genotypes. Clin Biochem. 2014;47:1052-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Murphy BP, Pang TY, Hannan AJ, Proffitt TM, McConchie M, Kerr M, Markulev C, O’Donnell C, McGorry PD, Berger GE. Vascular endothelial growth factor and brain-derived neurotrophic factor in quetiapine treated first-episode psychosis. Schizophr Res Treatment. 2014;2014:719395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Sotiropoulou M, Mantas C, Bozidis P, Marselos M, Mavreas V, Hyphantis T, Antoniou K. BDNF serum concentrations in first psychotic episode drug-naïve schizophrenic patients: associations with personality and BDNF Val66Met polymorphism. Life Sci. 2013;92:305-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Ruiz de Azua S, Matute C, Stertz L, Mosquera F, Palomino A, de la Rosa I, Barbeito S, Vega P, Kapczinski F, González-Pinto A. Plasma brain-derived neurotrophic factor levels, learning capacity and cognition in patients with first episode psychosis. BMC Psychiatry. 2013;13:27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Katsuki A, Hayashi K, Atake K, Tomita M, Nakamura J. Aripiprazole altered plasma levels of brain-derived neurotrophic factor and catecholamine metabolites in first-episode untreated Japanese schizophrenia patients. Hum Psychopharmacol. 2012;27:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | González-Pinto A, Mosquera F, Palomino A, Alberich S, Gutiérrez A, Haidar K, Vega P, Barbeito S, Ortiz A, Matute C. Increase in brain-derived neurotrophic factor in first episode psychotic patients after treatment with atypical antipsychotics. Int Clin Psychopharmacol. 2010;25:241-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Rizos EN, Michalopoulou PG, Siafakas N, Stefanis N, Douzenis A, Rontos I, Laskos E, Kastania A, Zoumpourlis V, Lykouras L. Association of serum brain-derived neurotrophic factor and duration of untreated psychosis in first-episode patients with schizophrenia. Neuropsychobiology. 2010;62:87-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Jindal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naïve first episode schizophrenia. Schizophr Res. 2010;119:47-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Goto N, Yoshimura R, Kakeda S, Moriya J, Hayashi K, Ikenouchi-Sugita A, Umene-Nakano W, Hori H, Ueda N, Korogi Y. Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Hum Psychopharmacol. 2009;24:639-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Rizos EN, Siafakas N, Stefanis N, Douzenis A, Kontaxakis V, Laskos E, Kastania A, Zoumbourlis V, Lykouras L. Association of serum BDNF and val66met polymorphism of the brain-derived neurotrophic factor in a sample of first psychotic episode patients. Psychiatriki. 2009;20:297-304. [PubMed] [Cited in This Article: ] |
| 21. | Chen da C, Wang J, Wang B, Yang SC, Zhang CX, Zheng YL, Li YL, Wang N, Yang KB, Xiu MH. Decreased levels of serum brain-derived neurotrophic factor in drug-naïve first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berl). 2009;207:375-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Rizos EN, Rontos I, Laskos E, Arsenis G, Michalopoulou PG, Vasilopoulos D, Gournellis R, Lykouras L. Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1308-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Palomino A, Vallejo-Illarramendi A, González-Pinto A, Aldama A, González-Gómez C, Mosquera F, González-García G, Matute C. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr Res. 2006;86:321-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Pirildar S, Gönül AS, Taneli F, Akdeniz F. Low serum levels of brain-derived neurotrophic factor in patients with schizophrenia do not elevate after antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:709-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Hambrecht M, Häfner H. Substance abuse and the onset of schizophrenia. Biol Psychiatry. 1996;40:1155-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 205] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | D’Souza DC, Pittman B, Perry E, Simen A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology (Berl). 2009;202:569-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Numata S, Ueno S, Iga J, Yamauchi K, Hongwei S, Ohta K, Kinouchi S, Shibuya-Tayoshi S, Tayoshi S, Aono M. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism in schizophrenia is associated with age at onset and symptoms. Neurosci Lett. 2006;401:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Decoster J, van Os J, Kenis G, Henquet C, Peuskens J, De Hert M, van Winkel R. Age at onset of psychotic disorder: cannabis, BDNF Val66Met, and sex-specific models of gene-environment interaction. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:363-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Aas M, Haukvik UK, Djurovic S, Tesli M, Athanasiu L, Bjella T, Hansson L, Cattaneo A, Agartz I, Andreassen OA. Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. J Psychiatr Res. 2014;59:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |