Published online Jun 9, 2015. doi: 10.5497/wjp.v4.i2.180
Peer-review started: December 2, 2014
First decision: February 7, 2015
Revised: March 9, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: June 9, 2015
Processing time: 199 Days and 7 Hours
Pancreatic ductal adenocarcinoma is an aggressive and devastating disease associated with poor survival outcomes. Even though significant advances have been made towards understanding the intricate pathology of this cancer, several important aspects remain unknown. Recently, key genetic mutations within the tumour have been identified, but the exact role they play in tumourigenesis has yet to be determined. For many years, the micro-tumour environment and stroma was thought to aid proliferation but there is now emerging research that suggests the contrary. Several novel targeted agents in pre-clinical and early clinical studies have been promising but it remains to be seen whether they will have a significant impact on patient outcomes. In this review we discuss the unique nature of pancreatic cancer biology, current treatment options and summarise the latest results from pre-clinical and clinical research. We also discuss the future strategies that are needed to improve outcomes for this disease.
Core tip: Pancreatic ductal adenocarcinoma is a cancer with several significant genetic aberrations that have recently been identified by international research efforts. Despite these findings, standard therapy for advanced disease consists primarily of chemotherapy. In the last few years two new chemotherapy regimens, FOLFIRINOX and Gemcitabine/Nab-paclitaxel, have demonstrated survival benefits in large phase III trials resulting in a change to current practise. However, the advent of targeted treatments has not yet had a significant impact in this disease compared with other malignancies. Current research strategies include developing therapies directed towards the RAS-RAK-MEK pathway, PI3K-AKT-mTOR pathway, notch pathway and immunotherapies to name but a few, with several clinical trials underway. It is likely that the heterogeneous nature of pancreatic cancer necessitates a more personalised approach to management with targeted treatment guided by predictive biomarkers.
- Citation: Thillai K, Sarker D, Ross P. Progress in pancreatic cancer therapeutics: The potential to exploit molecular targets. World J Pharmacol 2015; 4(2): 180-192
- URL: https://www.wjgnet.com/2220-3192/full/v4/i2/180.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i2.180
Pancreatic ductal adenocarcinoma (PDAC) is the 5th leading cause of cancer related mortality worldwide. Despite significant research efforts, 5-year survival for all stages remains stagnant at 3%-5%[1,2]. More than 80% of patients present with inoperable or advanced disease, so there remains an urgent need for more effective systemic treatments[3]. Although newer combination chemotherapy regimens offer improved survival outcomes, therapeutic options are limited and do not fully exploit the unique biology of the disease. To date no predictive or prognostic biomarker has been validated for use. This review will discuss current management and emerging therapeutic concepts.
Pancreatic cancer is a genetic disease, the biology of which is both intricate and highly heterogeneous. Several extensive genomic studies have confirmed that the development of PDAC results from several complex genetic aberrations with mutations in both oncogenes and tumour suppressor genes[4]. The progression from the pre-malignant dysplastic cellular transformations to the final development of PDAC is associated with increasing mutational changes, confirming the importance of such genetic variations in cancer development. Nearly all pancreatic cancers harbour a KRAS mutation and the majority are also associated with inactivation of CDKN2a/INK4a, TP53 and DPC4/SMaD4[4-7]. Several groups have aimed to accurately depict the genomics of PDAC with each reporting numerous activating mutations. Importantly, there was heterogeneity amongst the different pancreatic tumours with several key pathways responsible for cancer progression differing between patient samples[7]. Clonal mutations found in metastatic lesions were identified in primary cancers but due to the unstable genetic nature of PDAC, these initial mutations continued to evolve resulting in heterogeneity amongst the different metastatic deposits in the same patient. This complex genetic landscape results in an aggressive pathology, often refractory to treatment resulting in poor survival outcomes.
A recent addition to the understanding of PDAC comes from recent studies that have identified a group of pancreatic cancer cells that display stem cell properties[4,8]. These cells appear to have the ability of self-renewal and asymmetric division. Preliminary data suggests that patients with tumours containing cancer stem cells (CSCs) are associated with poorer overall survival (OS). The identification of CSCs and the signalling pathways that they regulate, has led to newer therapeutic targets such as WnT, Hedgehog and Notch. Further research is needed to see whether these can be successfully exploited to produce meaningful clinical outcomes.
Despite promising pre-clinical studies, several chemotherapeutics and targeted agents have failed to reproduce positive results in patients (Table 1). One explanation for this relates to the complex micro-tumour environment that surrounds the cancer cells and the difficulties replicating this in-vitro. A significant bulk of the pancreatic tumour comprises not of malignant cells but of the encompassing dense fibrotic stromal matrix[9]. This micro-tumour environment results from the extensive desmoplastic reaction seen in PDAC and consists of an abundant extracellular matrix, pancreatic stellate cells, fibroblasts, immune cells, inflammatory cells and vasculature all of which were previously thought to aid proliferation, invasion and metastatic spread whilst also preventing adequate drug delivery leading to chemotherapy-resistance[10-14]. The success of nab-paclitaxel (as discussed later) appear to manipulate the distinct characteristics of the stroma for therapeutic benefit[15].
| Datepublished | Target | Ref. | Sample size (n) | Treatment | OS (mo) | P value |
| 2001 | MMP | Bramhall et al[100] | 414 | Marimastat and Gem vs Gem | 5.4 | 0.95 |
| 5.4 | ||||||
| 2004 | FT | Van Cutsem et al[31] | 688 | Tipifarnib and Gem vs Gem + Placebo | 5.9 | 0.75 |
| 6.3 | ||||||
| 2009 | EGFR | Moore et al[61] | 569 | Erlotinib and Gem vs Gem | 6.2 | 0.038 |
| 5.9 | ||||||
| 2008 | EGFR/VEGF | Van Cutsem et al[88] | 301 | Gem, Erlotinib and Bevacizumab vs Gem, Erlotinib and Placebo | 7.1 | 0.2087 |
| 6.0 | ||||||
| 2010 | VEGF | Kindler et al[87] | 535 | Gem and Bevacizumab vs Gem and Placebo | 5.8 | 0.95 |
| 5.9 | ||||||
| 2010 | EGFR | Philip et al[75] | 745 | Gem vs Gem and cetuximab | 5.9 | 0.23 |
| 6.3 | ||||||
| 2011 | VEGF | Kindler et al[101] | 630 | Axitinib and Gem vs Gem | 8.5 | 0.54 |
| 8.3 | ||||||
| 2012 | VEGF, BRAF, PDGFR-B | Gonçalves et al[102] | 104 | Sorafenib and Gem vs Gem | 8.0 | 0.23 |
| 9.2 |
However recent emerging research using genetically modified mouse models suggest that depletion of the stroma (by genetic or pharmacological targeting of the Hedgehog[16] pathway) results in an unexpected increase in tumour vascularity and proliferation, thereby resulting in more aggressive tumours with reduced survival[17]. Furthermore, transgenic mice with the ability to delete αSMA+ myofibroblasts in pancreatic cancer also demonstrated reduced survival[18]. Both studies suggest that rather than a promoter of cancer growth, the stroma (or at least part of it) may paradoxically act to suppress proliferation and angiogenesis thus targeting the stroma should be performed with caution. An intricate and crucial part of PDAC, further research into the stroma is needed in order to exploit its presence for therapeutic benefit.
Until recently the standard treatment for inoperable or metastatic disease was with the nucleoside analogue gemcitabine. This was based on the results of a phase III trial in 1997 of 126 patients with advanced PDAC. Patients were randomised to receive gemcitabine (gemcitabine 1000 mg/m2 weekly × 7 followed by 1 wk of rest, then weekly × 3 every 4 wk thereafter), or to fluorouracil (5-FU) (600 mg/m2 once weekly)[19]. Both arms continued treatment until progression or unacceptable toxicities and the primary end point was clinical benefit, measured using a combined score of pain, performance status (PS) and weight loss. Clinical benefit response was experienced by 23.8% of gemcitabine-treated patients compared with 4.8% of 5-FU-treated patients (P = 0.0022). There was also a modest survival benefit with gemcitabine with a 12-mo survival of 18% vs 2% (P = 0.0025) with a median OS of 5.6 m for patients treated with gemcitabine and 4.4 mo for those with 5-FU. Treatment was well tolerated and gemcitabine became standard treatment for inoperable and advanced disease.
Numerous clinical trials with various chemotherapy agents combined with gemcitabine ensued (Table 2) and following several disappointing outcomes, the majority of preclinical work focused on developing new targeted treatments. However, the most significant advances in PDAC management were with two new chemotherapy combinations that have recently demonstrated benefits over gemcitabine in large phase III trials and thus changed current practise. The combined treatment of oxaliplatin, irinotecan, leucovorin and fluorouracil (FOLFIRINOX) was associated with a median OS of 11.1 mo compared with 6.8 mo in patients treated with gemcitabine alone (HR for death 0.57, 95%CI: 0.45-0.73, P≤ 0.001)[20,21]. This phase III trial of 342 patients with PS 0 or 1 also demonstrated increases in median progression free survival (PFS) (6.4 m vs 3.3 m, P≤ 0.001) and objective response rate (ORR) (31.6 vs 9.4, P≤ 0.001)). Approved for use in first line metastatic disease, in practise this regimen is generally reserved for patients with an excellent performance status as unsurprisingly, toxicity was also significantly increased with this 3-drug combination. More recently a phase III trial compared combined nab-paclitaxel and gemcitabine with gemcitabine alone in patients with metastatic disease[22]. Median OS was 8.5 mo with the combination chemotherapy and 6.7 mo with gemcitabine (95%CI: 0.62-0.83, P < 0.001). ORR was significantly increased at 23% vs 7% (P < 0.001), leading to interest in the potential use as a means of down staging locally advanced disease. A further pre-specified sub-group analysis concluded that baseline Karnofsky score (KS), presence of liver metastases, age and number of metastatic sites were independent prognostic factors for OS and PFS[23]. Common adverse events of grade 3 or higher included neutropenia (38% in nab-paclitaxel and gemcitabine arm and 27% in the gemcitabine arm), fatigue (17% and 7%) and neuropathy (17% and 1%). Nab-paclitaxel is a colloidal suspension of 130 nm particles homogenised in human serum albumin that is bound to paclitaxel. Pancreatic cancers are known to overexpress Secreted protein acidic and rich in cysteine (SPARC) and nab-paclitaxel improves efficacy via SPARC-albumin binding[24]. Pre-clinical models have confirmed that SPARC overexpression in the stroma promotes cell invasion and metastatic spread. Higher levels of SPARC appeared to correlate with improved survival in the original phase I/II trial of gemcitabine and nab-paclitaxel (mOS was 17.8 m in the high SPARC group compared with 8.1 m in the low SPARC group, P = 0.431). Further research is needed to confirm whether SPARC has the potential to be used as a predictive marker. The recently reported results from a prospective randomised adjuvant study have also suggested the prognostic significance of over-expressed SPARC in patients undergoing resection with curative intent. Disease free survival (DFS) was 7.4 mo in patients with higher levels of SPARC compared to 12.1 m in those with lower levels (P = 0.041) and OS was 14.1 and 25.6 m respectively (P = 0.011)[25,26]. Without a direct head- to -head trial of both combination chemotherapy regimens, it is difficult to ascertain whether FOLFIRINOX or gemcitabine and nab-paclitaxel is superior and both are now standard practise. However single agent gemcitabine remains treatment of choice for those patients that are not suitable for combination therapy.
| Date published | Regimen | Ref. | Sample size (n) | Median OS (mo) | P value |
| 2001 | Gem vs Gem + 5FU | Berlin et al[103] | 322 | 5.4 | 0.09 |
| 6.7 | |||||
| 2004 | Gem vs Gem + Irinotecan | Rocha Lima et al[104] | 360 | 6.6 | 0.789 |
| 6.3 | |||||
| 2005 | Gem vs GemOx | Louvet et al[105] | 326 | 7.1 | 0.13 |
| 9.0 | |||||
| 2007 | Gem vs Gem + cape | Herrmann et al[106] | 319 | 7.2 | 0.234 |
| 8.4 | |||||
| 2006 | Gem vs Gem + Irinotecan | Stathopoulos et al[107] | 145 | 6.4 | 0.970 |
| 6.5 | |||||
| 2006 | Gem vs Gem + Cisplatin | Heinemann et al[108] | 195 | 6.0 | 0.15 |
| 7.5 |
Approximately 30% of all patients with solid malignancies have tumours that exhibit oncogenic Ras mutations[27]. In PDAC this figure is much higher as an excess of 95% have a small GTPase KRAS mutation resulting in a dominant activated form. These mutations cause the protein to be constitutively activated, which leads to aberrant down-stream signalling and increased proliferation[28]. Following the discovery of the Ras family, a concerted effort was made to develop agents that could block mutated Ras function with little success.
As KRAS requires binding to the plasma membrane via farnesylation or geranylgeranylation in order to become activated, several farnesyltransferase inhibitors (FFIs) have been developed but have proved ineffective in clinical trials. Two phase II trials using FFIs were negativeand a randomised doubled blind phase III trial of 688 patients comparing gemcitabine with or without the FFI tipifarnib, demonstrated no significant survival benefit in the combination arm compared with standard treatment[29-31]. A further study demonstrated that binding of mammalian PDEδ to KRAS using small molecule inhibitors can suppress oncogenic RAS signalling by virtue of selective binding to the prenyl-binding pocket of PDEδ and in PDAC cell lines resulted in reduced cell proliferation[32]. Other approaches include the development of small molecules that target son of sevenless (SOS) mediated nucleotide exchange and subsequently target KRAS[33] and recently KRASG12C inhibitors have demonstrated therapeutic potential by allosterically allowing KRAS to favour GDP over GTP[34]. Another recent approach to targeting KRAS is by the combined MEK/BCL-XL inhibition, a method developed after identification in a pooled shRNA screen[35]. This combination resulted in significant apoptosis in several KRAS mutated cell lines.
A recent pre-clinical study demonstrated a novel way of targeting KRAS in transgenic mouse models using an siRNA delivery system (Local drug EluteR or LODER)[36]. This model capitalises on the effects of siRNA and knockdown of KRAS, but via an innovative platform of a controlled and prolonged delivery for therapeutic benefit. The LODER against KRAS (siG12D LODER) decreased KRAS levels in pancreatic cancer cell lines resulting in reduced proliferation and epidermal-mesenchymal transition. Within in-vivo models, the growth of human pancreatic cancer cells was impaired and mouse survival was increased compared to controls. A phase 1 study in patients with locally advanced disease treated with siG12D LODER is on-going[37], and a further phase II study of siG12D LODER in combination with chemotherapy plans to open early next year[38]. Whilst these results are promising in the pursuit of an anti-KRAS therapy, it remains to be seen whether this can be translated in to an efficacious treatment in clinical trials.
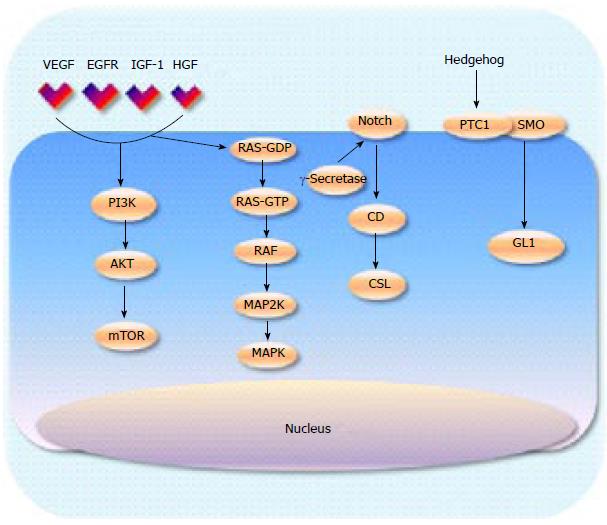
With the limited success of inhibiting Ras, efforts have moved towards targeting downstream signalling activity. There are two main pathways that have been extensively interrogated, mitogen-activated protein kinases (MAPK) and phosphoinositide 3 kinase (PI3K) signalling (Figure 1). BRaf inhibitors, such as vemurafenib, work downstream from Ras and have had considerable success in Raf mutant tumours such as melanoma[39]. However, evidence now supports that there is a paradoxical up regulation of MAPK signalling when Raf is inhibited in KRAS mutated tumours[40]. In pancreatic cancer where Raf is wild type and Ras is nearly always mutated, Raf inhibitors create feedback activation of the MAPK signalling pathway therefore it is likely that targeting downstream by MEK inhibition will offer more promising results. Phase I/II clinical trials of various MEK inhibitors in combination with gemcitabine are currently underway following positive pre clinical work[41,42], Results from a phase IIa trial of 60 patients treated with gemcitabine in combination with the allosteric oral Mek1/2 inhibitor refametinib were presented at ASCO 2104. The best result was partial response in 35% of patients with median duration of response at 3.8 mo (117 d 95%CI: 83-265). Time to progression was 7.4 mo[43,44]. KRAS mutations were identified in 39 patients (65%) and the results suggested a trend towards improved survival outcomes in patients with KRAS wild type tumours. The OS for the KRAS mutant subgroup was 6.6 mo compared with 18.2 mo (HR = 0.27).
PI3K is an enzyme that lies downstream from RAS and is responsible for the activation of AKT, which in turn leads to activation of mammalian target of rapamycin (mTOR) (Figure 1). In normal tissue the PI3K-AKT pathway inhibits apoptosis and cell proliferation, thus deregulation of this pathway leads to unregulated cell[45]. Several PI3K inhibitors have been developed and are currently being investigated in a number of malignancies. Preliminary studies using transgenic mice have demonstrated reduced pancreatic tumour growth when PI3K is inhibited and therefore PI3K remains a valid therapeutic target that warrants further attention[46]. The dual PI3K/PDK inhibitor rigosertib, has demonstrated safety and some efficacy when combined with gemcitabine in pre-treated patients with advanced disease[47] and a phase III trial in combination with chemotherapy is underway[48].
mTOR has been identified as a critical effector in cell signalling and the drug everolimus, an oral inhibitor of mTOR, has had success against solid tumours such as metastatic renal cell cancers and breast cancers, but also in pancreatic neuroendocrine tumours[49-51]. Preclinical studies showed that inhibition of the mTOR pathway suppressed proliferation in pancreatic cancer cell lines[52]. However in a phase II study of 33 patients with gemcitabine refractory metastatic PDAC, there were no complete or partial responses and median PFS was 1.8 mo and OS was 4.5 mo[53]. A phase 1 trial combining gemcitabine with temsirolimus, another mTOR inhibitor, resulted in significant toxicity without any partial or complete responses. The commonly used anti-diabetic drug metformin is also known to inhibit mTOR and epidemiological studies have linked metformin use with reduced risk of developing malignancies[54,55]. Metformin is now the focus of a clinical trial and is being used in combination with chemotherapy[56]. Novel agents that comprise of both mTOR complex 1 and 2 inhibitors (mTORC1/2) have shown promising efficacy in cancer cells in vitro. One such agent, INK-128, led to pancreatic cancer cell apoptosis and necrosis in vitro. Furthermore INK-128 resulted in increased sensitivity of pancreatic cancer cells to gemcitabine suggesting potential benefit when used in combination with chemotherapy[57]. Other positive results have been reported with dual PI3K/mTOR kinase inhibitors in vitro[58]. Recently inhibitors against the p110δ isoform of PI3K demonstrated inactivation of regulatory T cells leading to CD8+ cytotoxic cells and subsequent tumour regression in murine models[59]. Despite limited results in clinical trials thus far, recent pre-clinical efforts are more promising and the PI3K/AKT/mTOR signalling cascade remains an important pathway for future research.
The epidermal growth factor receptor (EGFR) has also emerged as an attractive therapeutic target for many malignancies. EGFR is a member of the erbB/human EGFR family of tyrosine kinases and when bound to a ligand, a conformational change is induced leading to dimerisation with other receptors[60]. This results in the activation of several cascades including the Ras/MAP kinase pathway and the PI3K/Akt/mTOR pathway. Several small molecules have been developed that block EGFR with varying degrees of success. The only targeted drug to be approved for the management of advanced PDAC so far is the tyrosine kinase inhibitor (TKI) erlotinib when administered in combination with gemcitabine. A phase III trial (PA.3) randomly assigned 569 patients with advanced disease to receive standard gemcitabine plus erlotinib (100 or 150 mg/d orally) or placebo[61]. The trial was double blinded with a primary end point of overall survival. The results showed a modest but significant survival benefit in the combination arm (6.24 m vs 5.91 m HR = 0.82, 95%CI: 0.69-0.99, P≤ 0.038), which led to FDA approval. Although the benefits appeared to be small, an unplanned retrospective subgroup analysis led the authors to hypothesise that patients who developed a skin rash on treatment experienced a higher disease control rate. Patients that were younger than 65 (P = 0.1) and those with a good PS (P = 0.03) were more likely to develop a rash. The median OS in patients with a grade 0, 1 or 2 rash were 5.3 m, 5.8 m and 10.5 m respectively with 1 year survival rates of 16%, 9% and 43% (P < 0.001). This was further assessed in a study correlating rash and survival outcomes, by analysing combined data from the PA.3 trial and a phase III trial using erlotinib in advanced non-small cell lung cancer (BR.21)[62,63]. They found that the presence of grade 2 or higher rash correlated with improvements in PFS and disease control. These findings were echoed in a retrospective study of 174 patients that found that high-severity rash was associated with longer OS[64].
However, molecular studies have not been able to identify EGFR and KRAS mutations as predictive biomarkers of survival benefit and no association between KRAS mutation or EGFR gene copy number with rash has been identified. Erlotinib in combination with capecitabine has also been shown to have some activity in gemcitabine refractory patients as evidenced by a phase II trial combining capecitabine and erlotinib in patients with advanced PDAC. The primary end point was response and this was found to be 10% of all 30 patients with a median OS of 6.5 mo[65]. A further phase III trial comparing combined capecitabine and erlotinib followed by gemcitabine on progression compared with gemcitabine and erlotinib followed by capecitabine is on-going[66].
An alternative anti EGFR TKI, gefitinib, demonstrated anti-proliferative effects in the pre clinical setting and this has translated to positive survival benefit in patients with non small cell EGFR mutated lung cancer[67]. Gefitinib combined with gemcitabine has been assessed in a phase II trial of 53 patients with locally advanced or metastatic PDAC. Patients were treated with gefitinib (250 mg) once daily and gemcitabine at the standard dose and schedule. 6 mo PFS was 30% with a median PFS of 4.1 mo. The 1-year survival rate was measured at 7.3 mo. Whilst these results were comparable to the PA 3 trial, there has yet to be a randomised trial of gefitinib to demonstrate significant benefit over single agent gemcitabine[68].
The anti-EGFR antibody cetuximab has shown significant clinical activity in both colorectal cancers and head and neck tumours in patients with wild type KRAS[69,70]. Despite the majority of patients with pancreatic cancer having KRAS mutations, preclinical activity suggested that it might be a useful therapy in advanced PDAC due to EGFR overexpression[71-73]. A phase II trial evaluated gemcitabine and cetuximab in 41 treatment-naïve patients stratified according to EGFR expression using immunohistochemistry (4 patients were 1+, 20 patients were 2+ and 17 patient were 3+)[74]. Cetuximab was administered at a loading dose of 400 mg/m2 followed by 250 mg/m2 weekly and gemcitabine was administered 1000 mg/m2 weekly for 7 wk and then 100 mg/m2 every week for three weeks followed by a week’s rest. Five patients achieved a partial response (12.5%) and 26 patients (63.4%) had disease stability. Median TTP was 3.8 mo and the median OS was 7.1 mo. Survival at 1 year was 31.7%. Toxicities were as previously reported with cetuximab chemotherapy combinations, most notably rash (87.7%), nausea (61.0%), weight loss (58.5%) and diarrhoea (53.7%). Despite the promising results from this phase II trial, this was not reproduced in 2 phase III trials. The S0205 trial conducted by the southwest oncology group (SWOG) reported that in 766 patients treated with either gemcitabine or gemcitabine plus cetuximab, there was no survival benefit seen in the combination arm[75]. A further trial combining gemcitabine and cisplatin with or without the addition of cetuximab, recruited 40 patients. Seven patients had a documented response in the antibody arm compared to 5 in the control arm but again no survival benefit was seen with cetuximab[76]. A further negative phase II trial with gemcitabine and oxaliplatin with the addition of cetuximab recruited 64 patients. Patients received a combination of gemcitabine at 100 mg/m2 on day 1 with oxaliplatin at 100 mg/m2 on day 2, every 2 wk. Cetuximab was administered at a loading dose of 400 mg/m2 followed by weekly dose of 250 mg/m2. Although well tolerated, the findings (response rate 33%, median time to PFS 3.9 mo and OS 7.1 mo) were not superior to previously seen results using the chemotherapy combination alone[77]. The results of a phase II trial presented at ASCO 2013, portrayed a significant survival benefit at 1 year with gemcitabine combined with the anti-EGFR antibody nimotuzumab compared to gemcitabine alone (34.4% vs 19.5%, P = 0.034, HR = 0.69) and the combination was well tolerated[78]. A phase II study of nimotuzumab in pre-treated patients with advanced PDAC was also encouraging and a randomised placebo controlled phase IIb/IIIa study comparing the combination of gemcitabine and nimotuzumab compared to gemcitabine and placebo has recently closed to recruitment and the results are awaited[78].
Although EGFR remains a critical receptor in pancreatic cell proliferation and metastatic spread, with the exception of the modest benefits seen in the PA3 trial, there have not been any positive results with EGFR targeted therapy in large randomised trials. Whilst monoclonal antibodies that target EGFR have demonstrated efficacy in other solid tumours, its distribution within pancreatic cancer cells is not well known and may be an explanation for poor outcomes. It is also possible that the optimum doses and methods of drug delivery have not yet been elucidated. With regards to erlotinib and gefitinib, the excellent results that have been demonstrated in several large clinical trials in lung cancer have not been reproduced in PDAC and are likely due to the lack of activating mutations seen in these tumours. There is not enough evidence to suggest that even in those with an activating mutation, this can predict response to anti-EGFR therapy. Thus disappointingly expression or mutation of EGFR has not emerged so far as a predictive or prognostic biomarker[79-81]. Unlike lung and colon tumours KRAS mutation is not mutually exclusive with EGFR activation. Initiation of KRAS mutated PDAC appears to be dependent on EGF activation and a recent study reported that EGF inhibition has limited therapeutic benefit in tumours with p53 inactivation[82]. The study hypothesised that p53 loss might “reactivate” the PI3K/AKT and the STAT pathway independent from EGF activation suggesting EGFR inhibitors may only be of clinical benefit in patients with p53 wild type tumours.
Angiogenesis describes the process by which a tumour initiates the formation of new vessels through remodelling of existing vasculature[83]. Once the “angiogenic switch” is initiated, the complex process of new vessel formation begins and subsequently plays a key role in tumour growth[84]. VEGF is vital to angiogenesis and is therefore a potential target in many tumour types with variable outcomes in clinical trials[85]. Anti-VEGF antibodies have been used without much success in pancreatic cancer. Bevacizumab, which offers improved outcomes in colorectal and ovarian cancer, is a monoclonal antibody that decreases the formation of new blood vessels in vivo and improves drug delivery to the cancer cell. A phase II trial of bevacizumab and gemcitabine in patients with advanced PDAC, demonstrated that in 52 patients, 19% had a partial response and 48% had stable disease[86]. The median OS was 8.8 mo with a 6-mo survival of 77%. This led to 2 phase III trials, both of which were disappointingly negative. The CALGB 80303 study treated patients with gemcitabine with or without bevacizumab[87,88]. 602 patients were enrolled and both overall response rate and 1 year survival outcomes failed to reach statistical benefit in the combination arm. The AViTA trial, comparing the combination of gemcitabine and erlotinib with the addition of bevacizumab was also negative[88]. Despite the changes in the vasculature seen in patients treated with these drugs, no benefit has been shown when targeting VEGF and the exact mechanism of failure remains unknown but is likely to be in part due to the hypovascularity of the surrounding stroma[11].
Interest in immunotherapy has had a recent resurgence following the results of several positive clinical trials in solid malignancies including melanoma and prostate cancer[89,90]. Success has been more modest in pancreatic cancer although several newer agents remain under investigation. Based on the understanding that the innate immune system can distinguish between cancer cells and “normal self”, exploitation of the immune system has been a topic of research for several decades. Not only do immune-deficient mice develop malignancies, evidence has also shown that patients with cancer develop B and T cells that can recognise antigens released by pancreatic tumour cells. The immune response created by the patient is invariably unsuccessful at eliminating malignancy but this reaction can be enhanced for therapeutic gain. Theoretically, immunotherapy should be active in pancreatic cancer, as the dense stroma is enriched with immune cells such as T cell and macrophages.
A recent positive trial presented at the Gastrointestinal Cancers Symposium in 2014 demonstrated significant survival benefit when combining two specific anti-cancer vaccines compared with monotherapy. GVAX is a vaccine made from 2 pancreatic cancer cell lines that have been irradiated to secrete granulocyte-macrophage colony-stimulating factor causing stimulation of the immune system[91]. Administered intra-dermally after low dose cyclophosphamide, it inhibits regulatory T cells. CRS-207 is made of live-attenuated Listeria monocytogenes engineered to stimulate an immune response against a protein called mesothelin that is expressed at high levels in pancreatic cancer cell lines. This phase II trial compared the combination of CRS-207 and GVAX with GVAX alone with positive outcomes. Ninety patients with pre-treated PDAC were randomly assigned at a ratio of 2:1 to be treated with 2 dose of CY/GVAX followed by 4 doses of CRS-207 or 6 doses of CY/GVAX every three weeks. The primary end point was OS with safety, clinical response and immune response secondary. At the interim analysis median OS was 6.1 m with the combination treatment compared with 3.9 m for GVAX therapy. (HR = 0.59, two sided Log Rank P = 0.03). One-year survival was doubled with combination treatment (24% vs 12%). Following the encouraging results from the interim analysis, crossover was allowed. Toxicities included fevers, rigors and lymphopaenia, but were minimal and were not cumulative. Several other studies are due to open comparing combination CY/GVAX and CRS-207 with chemotherapy in the second line setting or in combination with immune checkpoint inhibitors of programmed death 1 (PD1) and its ligand PD-L1. PD1, which is a T-cell co-inhibitory receptor, and PD-L1 have shown considerable responses in certain solid tumours including melanoma and lung cancers. An international phase 1 study using the intravenous anti-PD-L1 antibody treated 75 patients, 14 of whom had pancreatic cancer. Objective responses were seen in patients with non-small lung cancer, melanoma, renal and ovarian cancer but not in those with PDAC[92]. However there remains potential benefit with PD-1 in combination with other compounds. Recently the effects of PD-1 immunosuppression were enhanced when used in combination with chimeric antigen reception (CAR) T-cell therapy in Her2 transgenic mice. Further research with this combination is on going. A phase 1 study combining the agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine demonstrated tolerability and resulted in 4 out of 22 patients with advanced PDAC achieving a partial response suggesting that further clinical trials are warranted.
The results from a phase III trial assessing the GV1001 vaccine, a promiscuous class II epitope vaccine, recently reported no benefit when used in combination with gemcitabine and capecitabine compared to chemotherapy alone and was therefore terminated early[93]. Several other immunological treatments remain under review. The anti-CTLA4 antibody that has been approved for use in melanoma, also demonstrated no initial responders to therapy. However in this phase II trial of 27 patients, 1 patient had a significant delayed response[94]. Single agent ipilimumab has not been taken forward to a phase III trial but its safety when used in combination with gemcitabine is currently being assessed in an early phase trial[95]. As it has been suggested that immunotherapy is most successful in the absence of large disease burden, several clinical trials are assessing immunotherapy in the post-operative setting or as maintenance therapy following response to chemotherapy.
The hedgehog (Hh) pathway has been identified as another important signalling cascade in multiple cancers suggesting its potential as a therapeutic target. Two transmembrane proteins have been identified that activate the Hh signalling pathway, the tumour suppressor patched protein (PTCI) and smoothened (SMO) an oncogenic protein[96-98]. Pre-clinical studies have established that human pancreatic stellate cells (as seen in the stroma) express high levels of smoothened protein and low levels of Hh ligands unlike the pancreatic cancer cells, which demonstrate the converse expression pattern[11]. The majority of Hh inhibitors that have been developed target SMO. In transgenic Kras mutated mice the administration of a Hh inhibitor IPI-926 depleted the surrounding stroma enhancing the drug delivery of gemcitabine[11]. A phase Ib trial of IPI-926 in combination with gemcitabine demonstrated acceptable tolerability in 16 patients with untreated metastatic PDAC. Common AEs included fatigue, thrombocytopenia, anaemia, nausea, diarrhoea, vomiting and dose reductions of IPI-926 were required in 3 patients. DR of gemcitabine occurred in 11 patients. Five sixteenths (31%) had a radiological response while median PFS was more than 7 mo with 74% patients alive after 6 mo of entry in to the study. Whilst these results were promising, a phase II trial was terminated early at the interim analysis as patients in the combination arm experienced worse outcomes than those on single agent gemcitabine[99]. These disappointing results may be partly explained by the results from recent pre-clinical studies suggesting the importance of the stroma (as discussed earlier) where depletion led to increased tumour growth[16,17].
However further research with Hh inhibitors are on-going. A single-arm study with the Hh inhibitor vismodegib combined with the chemotherapy regimen gemcitabine and nab-paclitaxel, presented an interim analysis at GI ASCO 2014[16]. Eighty percent of the 59 patients treated had stable disease or better. Median PFS was 5.5 mo and OS was 10 mo. Patient recruitment is on-going and based on the preliminary results, the final survival data is eagerly awaited.
The current prognosis for advanced PDAC remains poor, highlighting the urgent need for more effective systemic therapies. In order to develop targeted treatments and improve outcomes, research efforts needs to focus on three key areas; a greater understanding of the unique biology of PDAC and the key signalling pathways, comprehension of the unique desmoplastic reaction and micro-tumour environment, and the development of predictive and prognostic biomarkers. It may be that the future of pancreatic cancer treatment will see combining standard chemotherapy with targeted treatments to achieve better outcomes. It is likely that PDAC treatment will be dictated by the biology of the individual tumour rather than the “one shoe fits all”approach that is used today. The last few years have seen significant results towards this in the pre-clinical setting but it remains to be seen whether they can be translated into meaningful clinical outcomes.
P- Reviewer: Cao DF, Fillat C, Folsch UR, Gu DS S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | WHO. int. Cancer. International Childhood Cancer Day. 2015; Available from: http://www.who.int/cancer/en/. |
| 2. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1693] [Article Influence: 153.9] [Reference Citation Analysis (0)] |
| 3. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9851] [Article Influence: 820.9] [Reference Citation Analysis (4)] |
| 4. | Hidalgo M. New insights into pancreatic cancer biology. Ann Oncol. 2012;23 Suppl 10:x135-x138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 858] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 7. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3017] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 8. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2126] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 9. | Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 459] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Liss AS, Thayer SP. Therapeutic targeting of pancreatic stroma. Available from: http://www.ncbi.nlm.nih.gov/books/NBK98931/. |
| 11. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2528] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 12. | Michl P, Gress TM. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut. 2012;61:1377-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Neesse A, Krug S, Gress TM, Tuveson DA, Michl P. Emerging concepts in pancreatic cancer medicine: targeting the tumor stroma. Onco Targets Ther. 2013;7:33-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, Ellenrieder V, Jodrell DI, Tuveson DA. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63:974-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2014] [Cited by in RCA: 2074] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 16. | Jesus-Acosta D. A phase II study of vimodegib, a hedgehog (Hh) pathway inhibitor, combined with gemciatbine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). J Clinic Oncol. 2014;32 suppl 3:abstr 257. |
| 17. | Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1621] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 18. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 1883] [Article Influence: 171.2] [Reference Citation Analysis (1)] |
| 19. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 20. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5586] [Article Influence: 399.0] [Reference Citation Analysis (1)] |
| 21. | Papadatos-Pastos D, Thillai K, Rabbie R, Ross P, Sarker D. FOLFIRINOX - a new paradigm in the treatment of pancreatic cancer. Expert Rev Anticancer Ther. 2014;14:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Von Hoff DD, Goldstein D, Renschler MF. Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med. 2014;370:479-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, Scheithauer W, Ramanathan RK, Goldstein D, Penenberg DN. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, Castrillon DH, Sage EH, Puolakkainen P, Bradshaw AD. Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech. 2010;3:57-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Sinn M, Sinn BV, Striefler JK, Stieler J, Pelzer U, Prinzler J, Neuhaus P, Dietel M, Dörken B, Oettle H. SPARC in pancreatic cancer; Results from the CONKO-001 study. ASCO annual meeting 4016. J Clin Oncol. 2013;31 suppl:abstr 4016. |
| 26. | Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 869] [Article Influence: 62.1] [Reference Citation Analysis (2)] |
| 27. | Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730-733.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 539] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 28. | Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545-554. [PubMed] |
| 29. | Macdonald JS, McCoy S, Whitehead RP, Iqbal S, Wade JL, Giguere JK, Abbruzzese JL. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs. 2005;23:485-487. [PubMed] |
| 30. | Cohen SJ, Ho L, Ranganathan S, Abbruzzese JL, Alpaugh RK, Beard M, Lewis NL, McLaughlin S, Rogatko A, Perez-Ruixo JJ. Phase II and pharmacodynamic study of the farnesyltransferase inhibitor R115777 as initial therapy in patients with metastatic pancreatic adenocarcinoma. J Clin Oncol. 2003;21:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430-1438. [PubMed] |
| 32. | Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M, Hahn SA, Triola G, Wittinghofer A, Bastiaens PI. Small molecule inhibition of the KRAS-PDEδ interaction impairs oncogenic KRAS signalling. Nature. 2013;497:638-642. [PubMed] |
| 33. | Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, Fauber BP, Pan B, Malek S, Stokoe D. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA. 2012;109:5299-5304. [PubMed] |
| 34. | Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548-551. [PubMed] |
| 35. | Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM, Greninger P, Brown RD, Godfrey JT, Cohoon TJ. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23:121-128. [PubMed] |
| 36. | Zorde Khvalevsky E, Gabai R, Rachmut IH, Horwitz E, Brunschwig Z, Orbach A, Shemi A, Golan T, Domb AJ, Yavin E. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20723-20728. [PubMed] |
| 37. | Silenseed Ltd. Phase 1 - Escalating dose study of siG12D LODER (Local Drug EluteR) in patients with advanced adenocarcinoma of the pancreas and a single dose study of siG12D LODER (Local drug EluteR) in patients with operable adenocarcinoma of the pancreas. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01188785 NLM Identifier: NCT01188785. |
| 38. | Silenseed Ltd. A phase II study of siG12D LODER in combination with chemotherapy in paitents with unresectable locally advanced pancreatic cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01676259 NLM Identifier: NCT01676259. |
| 39. | Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5824] [Cited by in RCA: 5919] [Article Influence: 422.8] [Reference Citation Analysis (0)] |
| 40. | Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1258] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 41. | Hatzivassiliou G; GlaxoSmithKline. Study of GSK1120212 plus gemcitabine vs placebo plus gemcitabine in metastatic pancreatic cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01231581 NLM Identifier: NCT01231581. |
| 42. | Merck KGaA. Trial of gemcitabine with or without MSC1936369B in pancreatic cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01016483 NLM Identifier: NCT01016483. |
| 43. | Merck KGaA; Bayer. Combination with gemcitabine in advanced pancreatic cancer (BAGPAC). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01251640 NLM Identifier: NCT01251640. |
| 44. | Riess H, Van Laethem JL, Martens UM, Heinemann V, Michl P, Peeters M, Van Brummelen D, Weekes CD, Dueland S, Schmiegel WH. Phase II study of the MEK inhibitor refametinib (BAY 86-9766) in combination with gemcitabine in patietns with unresectable, locally advacned, or metastatic pancreatic cancer. 2014 ASCO annual meeting 4025. J Clin Oncol. 2014;32:5s (suppl; abstr 4129). |
| 45. | Falasca M, Selvaggi F, Buus R, Sulpizio S, Edling CE. Targeting phosphoinositide 3-kinase pathways in pancreatic cancer--from molecular signalling to clinical trials. Anticancer Agents Med Chem. 2011;11:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M, Hieber M, Arbeiter A, Klein S, Kong B. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 47. | Ma WW, Messersmith WA, Dy GK, Weekes CD, Whitworth A, Ren C, Maniar M, Wilhelm F, Eckhardt SG, Adjei AA. Phase I study of Rigosertib, an inhibitor of the phosphatidylinositol 3-kinase and Polo-like kinase 1 pathways, combined with gemcitabine in patients with solid tumors and pancreatic cancer. Clin Cancer Res. 2012;18:2048-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Onconova Therapeutics, Inc . Gemcitabine and ON 01910.Na in previously untreated metasatic pancreatic cancer (ONTRAC). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01360853 NLM Identifier: NCT01360853. |
| 49. | Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2368] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 50. | André F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 51. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2102] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 52. | Matsubara S, Ding Q, Miyazaki Y, Kuwahata T, Tsukasa K, Takao S. mTOR plays critical roles in pancreatic cancer stem cells through specific and stemness-related functions. Sci Rep. 2013;3:3230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 54. | Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804-10812. [PubMed] |
| 55. | Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1692] [Cited by in RCA: 1815] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 56. | Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA). Metformin combined with chemotherapy for pancreatic cancer(GEM). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01210911 NLM Identifier: NCT01210911. |
| 57. | Lou HZ, Weng XC, Pan HM, Pan Q, Sun P, Liu LL, Chen B. The novel mTORC1/2 dual inhibitor INK-128 suppresses survival and proliferation of primary and transformed human pancreatic cancer cells. Biochem Biophys Res Commun. 2014;450:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Tang JY, Dai T, Zhang H, Xiong WJ, Xu MZ, Wang XJ, Tang QH, Chen B, Xu M. GDC-0980-induced apoptosis is enhanced by autophagy inhibition in human pancreatic cancer cells. Biochem Biophys Res Commun. 2014;453:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Ali K, Soond DR, Piñeiro R, Hagemann T, Pearce W, Lim EL, Bouabe H, Scudamore CL, Hancox T, Maecker H. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 417] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 60. | Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 426] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 61. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2767] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 62. | Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913-3921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 63. | Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 4211] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 64. | Stepanski EJ, Reyes C, Walker MS, Satram-Hoang S, Leon L, Wojtowicz-Praga S, Miller PJ, Houts AC, Schwartzberg LS. The association of rash severity with overall survival: findings from patients receiving erlotinib for pancreatic cancer in the community setting. Pancreas. 2013;42:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, Enzinger PC, Kwak EL, Muzikansky A, Lawrence C. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787-4792. [PubMed] |
| 66. | Heinemann V. Capecitabine/erlotinib followed of gemcitabine versus gemcitabine/erlotinib followed of capecitabine. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://www.clinicaltrials.gov/ct2/show/NCT00440167 NLM Identifier: NCT00440167. |
| 67. | Zhou X, Zheng M, Chen F, Zhu Y, Yong W, Lin H, Sun Y, Han X. Gefitinib inhibits the proliferation of pancreatic cancer cells via cell cycle arrest. Anat Rec (Hoboken). 2009;292:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Fountzilas G, Bobos M, Kalogera-Fountzila A, Xiros N, Murray S, Linardou H, Karayannopoulou G, Koutras AK, Bafaloukos D, Samantas E. Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer: a phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation. Cancer Invest. 2008;26:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1373] [Article Influence: 91.5] [Reference Citation Analysis (1)] |
| 70. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 71. | Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, Evans DB, Abbruzzese JL, Hicklin DJ, Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6:1936-1948. [PubMed] |
| 72. | Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Uegaki K, Nio Y, Inoue Y, Minari Y, Sato Y, Song MM, Dong M, Tamura K. Clinicopathological significance of epidermal growth factor and its receptor in human pancreatic cancer. Anticancer Res. 1997;17:3841-3847. [PubMed] |
| 74. | Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22:2610-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 75. | Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 499] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 76. | Cascinu S, Berardi R, Labianca R, Siena S, Falcone A, Aitini E, Barni S, Di Costanzo F, Dapretto E, Tonini G. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncol. 2008;9:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Kullmann F, Hollerbach S, Dollinger MM, Harder J, Fuchs M, Messmann H, Trojan J, Gäbele E, Hinke A, Hollerbach C. Cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line metastatic pancreatic cancer: a multicentre phase II study. Br J Cancer. 2009;100:1032-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Strumberg D. Phase II, randomized, double-blind placebo-controlled trial of nimotuzumab plus gemcitabine compared with gemcitabine alone in patients (pts) with advanced pancreatic cancer (PC). J Clin Oncol. 2013;31:(suppl; abstr 4009). |
| 79. | Immervoll H, Hoem D, Kugarajh K, Steine SJ, Molven A. Molecular analysis of the EGFR-RAS-RAF pathway in pancreatic ductal adenocarcinomas: lack of mutations in the BRAF and EGFR genes. Virchows Arch. 2006;448:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Kwak EL, Jankowski J, Thayer SP, Lauwers GY, Brannigan BW, Harris PL, Okimoto RA, Haserlat SM, Driscoll DR, Ferry D. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin Cancer Res. 2006;12:4283-4287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 81. | Faller BA, Burtness B. Treatment of pancreatic cancer with epidermal growth factor receptor-targeted therapy. Biologics. 2009;3:419-428. [PubMed] |
| 82. | Navas C, Hernández-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 83. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [PubMed] |
| 84. | Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2462] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 85. | Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 791] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 86. | Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033-8040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 341] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 87. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 671] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 88. | Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 499] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 89. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11728] [Article Influence: 781.9] [Reference Citation Analysis (0)] |
| 90. | May KF, Gulley JL, Drake CG, Dranoff G, Kantoff PW. Prostate cancer immunotherapy. Clin Cancer Res. 2011;17:5233-5238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi TS, Springett GM, Morse M, Zeh H, Cohen DJ, Fine RL. A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAZ alone in patients with metastatic pancreatic adenocarcinoma; Updated results. J Clin Oncol. 2014;32:(suppl 3; abstr 177). |
| 92. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9844] [Article Influence: 757.2] [Reference Citation Analysis (0)] |
| 93. | Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 94. | Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 964] [Cited by in RCA: 969] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 95. | Northwestern University. Ipililmumab and gemcitabine hydrochloride in treating patients with stage III-IV or recurrent pancreatic cancer that cannot be removed by surgery. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01473940 NLM Identifier: NCT01473940. |
| 96. | McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1-114. [PubMed] |
| 97. | Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 989] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 98. | Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 447] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 99. | Infinity Pharmaceuticals, Inc . A study evaluating IPI-926 in combination with gemcitabine in patients with metastatic pancreatic cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT01130142 NLM Identifier: NCT01130142. |
| 100. | Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447-3455. [PubMed] |
| 101. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 102. | Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 103. | Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 529] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 104. | Rocha Lima CM, Sherman CA, Brescia FJ, Brunson CY, Green MR. Irinotecan/gemcitabine combination chemotherapy in pancreatic cancer. Oncology (Williston Park). 2001;15:46-51. [PubMed] |
| 105. | Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 714] [Article Influence: 35.7] [Reference Citation Analysis (1)] |
| 106. | Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 107. | Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, Potamianou A, Ziras N, Boukovinas J, Varthalitis J. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95:587-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 495] [Article Influence: 26.1] [Reference Citation Analysis (0)] |