Copyright
©The Author(s) 2015.
World J Pharmacol. Mar 9, 2015; 4(1): 144-159
Published online Mar 9, 2015. doi: 10.5497/wjp.v4.i1.144
Published online Mar 9, 2015. doi: 10.5497/wjp.v4.i1.144
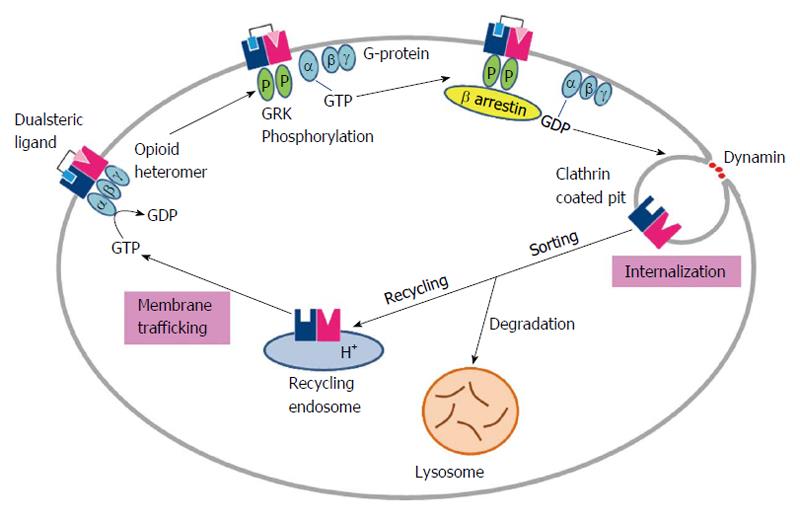
Figure 3 Dualsteric ligand induced regulation of opioid receptors heteromer.
Without agonist treatment, about 15% of the receptors are intracellular and G protein trimers are associated with each other with GDP bound to Gα subunits. Upon exposure to agonist, the receptor is activated and coupled to G proteins, triggering the exchange of GDP with GTP and dissociation of Gα from Gβγ subunits, which in turn activate down-stream effectors. Activation of the receptor also causes translocation and activation of GRKs, which phosphorylated the receptor in intracellular domains. Receptor phosphorylation enhances binding of β-arrestins, leading to uncoupling of receptors from G proteins. β-arrestins bind to clathrin and initiate movement of receptors into clathrin-coated pits, which are pinched by dynamin to become endosomes. Low pH in endosomes facilitates dissociation of agonists from the receptor and dephosphorylation of the receptor is believed to occur here. Internalized receptors are routed to two different pathways. Some are sorted to recycling endosomes and returned to plasma membranes. Alternatively, the receptors lead to degradation forming lysosomes. GRK: G-protein coupled receptor kinase; GDP: Guanosine-5'-diphosphate; GTP: Guanosine-5'-triphosphate.
- Citation: Mudgal A, Pasha S. Role of opioid receptor heterodimerization in pain modulation and tolerance development. World J Pharmacol 2015; 4(1): 144-159
- URL: https://www.wjgnet.com/2220-3192/full/v4/i1/144.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i1.144