Published online Dec 9, 2013. doi: 10.5497/wjp.v2.i4.107
Revised: August 9, 2013
Accepted: August 16, 2013
Published online: December 9, 2013
The aryl hydrocarbon receptor (AhR) was discovered more than three decades ago, and initially was characterized as a transcription factor with a role in xenobiotic metabolism. However, based on subsequent observations that AhR remains active under physiological conditions, exhibits constitutive expression during development, and has a high degree of conservation among species, it was hypothesized that AhR is responsible for functions in addition to its role in detoxification. Correspondingly, recent studies have elucidated novel physiological roles for this ligand-dependent transcription factor that link it to several pathways associated with disease development. In this review, studies are presented that support a role for AhR in cell proliferation, apoptosis, and immune homeostasis, thereby highlighting the therapeutic potential of this receptor for cancer and immune disorders.
Core tip: The goal of the present review was to discuss the role of the aryl hydrocarbon receptor (AhR) in cell proliferation and immune responses, and to highlight the potential for AhR to serve as a therapeutic target for cancer and immune diseases.
- Citation: Vega L, Elizondo G. Aryl hydrocarbon receptor as a new therapeutic target for cancer and immune disorders. World J Pharmacol 2013; 2(4): 107-114
- URL: https://www.wjgnet.com/2220-3192/full/v2/i4/107.htm
- DOI: https://dx.doi.org/10.5497/wjp.v2.i4.107
Discovery of the aryl hydrocarbon receptor (AhR) was the result of several efforts to understand the mechanism by which polycyclic aromatic hydrocarbons induce their own metabolism via aryl hydrocarbon hydroxylase (AHH) activity (now known to be mediated by CYP1A1). In addition, variations in the inducibility of AHH between mouse strains[1], as well as mutations present in mouse hepatoma cells[2], led to the discovery of the gene that encodes AhR[3].
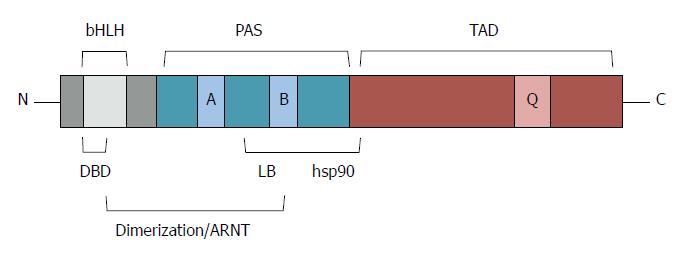
AhR is a member of the basic helix-loop-helix-Per-Arnt-Sim transcription factor family. The basic helix-loop-helix (bHLH) motif is located at the N-terminus of AhR, with two amphipathic α helices separated by a loop. This region is required for DNA binding and for protein dimerization. The Per-Arnt-Sim (PAS) domain is located at the C-terminus of the bHLH region. It function as a docking region for other PAS proteins such as AhR nuclear translocator (ARNT) and mediates binding of ligands and hsp90. Nuclear localization signal (NLS) and a nuclear export signal were located at the N-terminal region, while the C-terminal region of AhR contains a glutamine-rich transactivation domain recognized by several co-activators (Figure 1).
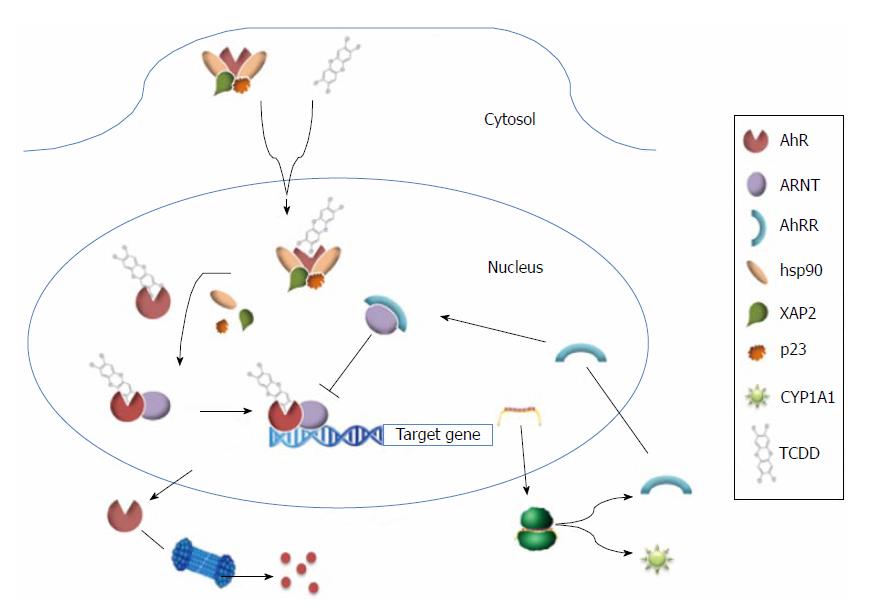
In its inactive form, AhR localizes to the cytoplasm where it can form a multiprotein complex with two molecules of hsp90, the co-chaperone protein, p23, and X-associated protein 2. However, upon binding of its ligands, the NLS of AhR is exposed and induces the translocation of AhR to the nucleus where it dimerizes with ARNT. The ligand-AhR-ARNT complex then binds dioxin-responsive elements, also known as xenobiotic-responsive elements, which contain the core sequence, 5’-GCGTG-3’. As a result, target genes of AhR are upregulated. Since dimerization of ARNT and AhR repressor (AhRR, also a bHLH protein) is competitive, AhR is negatively regulated. In addition, AhR is subject to degradation by the ubiquitin-proteasome 26S system (Figure 2).
AhR is highly conserved from invertebrates to vertebrates, and has three forms: AhR1, AhR2, and AhRR. Phylogenetic studies suggest that AhR emerged approximately 550 million years ago[4]. In mammals, AhR1 is the form expressed, and it has been found primarily in liver and lung tissues. Although, it has also been detected in several tissues and cell types, including brain, heart, kidney, muscle, placenta, thymus, spleen, macrophage, and lymphocytes.
Initially, AhR was studied as part of an adaptive chemical response since it mediates the toxic effects of environmental contaminants such as halogenated aromatic hydrocarbons (HAHs) and polycyclic aromatic hydrocarbons (PAHs). The prototype ligand for AhR is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which has the highest affinity for AhR and is the most potent of these compounds. However, based on the high degree of conservation across species and its constitutive expression, it is hypothesized the AhR may have additional roles in the cell. Indeed, in the recent years, several studies have elucidated novel physiological roles for this transcription factor in cell development[5], cell proliferation and apoptosis[6], circadian rhythmicity[7], cholesterol and glucose metabolism[8], immune system homeostasis[9], and more recently, in the ubiquitin-proteasome system[10,11].
Therefore, the goal of this review was to discuss the role of AhR in cell proliferation and immune responses, and to highlight the potential for AhR to serve as a therapeutic target for cancer and immune diseases.
AhR has been shown to mediate the toxic effects of HAHs and PAHs, including their carcinogenic forms. These compounds bind and activate AhR by inducing the expression of metabolizing enzymes, thereby promoting their own metabolism. In particular, PAHs such as benzo[a]pyrene are converted into highly mutagenic and carcinogenic metabolites by cytochrome P4501A1 and 1B1. The carcinogenic AhR dependent action of PAHs has been demonstrated using an AhR knockout mouse (AhR-null). Topical application of benzo[a]pyrene was found to produce skin tumors in wild-type mice, yet tumors did not develop in AhR-null mice that received the same application[12]. A clinical association between AhR and cancer has also been described as a result of accidental or occupational exposure to HAHs such as dioxins. In a follow-up study of a population accidentally exposed to dioxin, exposure to this compound increased the rates of mortality due to cancer[13,14]. Similarly, a cohort study reported that workers exposed to dioxins presented a higher risk for developing cancer[15] Although the carcinogenicity of dioxins is AhR-dependent, these compounds are rarely metabolized, and their mechanism(s) of action remain unknown.
In the last few years, a large number of studies have indicated that AhR, in addition to its xenobiotic-metabolizing function, has important roles in mediating cell proliferation and regulating apoptosis. In particular, AhR has been identified as a potential oncogene and tumor suppressor.
Overexpression of AhR has been detected in various types of human cancers, including pancreatic cancer[16], lung carcinoma[17], gastric cancer[17], and prostate cancer[18]. Similar results have been observed in rodent models. Expression of a constitutively active AhR in transgenic mice was found to induce stomach tumors[19], and to enhance the oncogenic potential of N-nitrosodiethylamine[20]. In vitro, overexpression of AhR in A549 cells was found to accelerate cell proliferation[21]. Activation of AhR in transformed cells in the absence of exogenous ligands has also been observed, with higher levels of AhR detected in nuclear fractions of HeLa cells[22]. However, in HaCaT cells, AhR localization appears to be influenced by cell density, with nuclear localization observed for AhR at low cell densities, and cytoplasmic localization observed when cells are confluent[23]. Taken together, these results support a role for AhR overexpression in tumor development.
Activation of AhR by exogenous ligands has also been shown to increase cell proliferation. For example, treatment of human mammary epithelial cells with benzo[a]pyrene increases intracellular levels of Ca2+, thereby accelerating cell proliferation[24]. The authors hypothesize that this effect may be mediated by epidermal grown factor receptor (EGFR), and the combination of these changes promote human breast cancer. Similarly, activation of AhR by TCDD in colon cancer cells induces EGFR phosphorylation and cell proliferation, and small interfering RNA targeting AhR abolished this effect[25]. Consequently, these data suggest that AhR may promote cell proliferation via activation of EGFR.
AhR also stimulates cell proliferation by interacting with other transcription factors. In particular, NF-κB-RelA/AhR interaction transactivates c-myc gene promoter by binding at a novel NF-κB-RelA/AhR response element[26]. Induction of c-myc then enhances cell proliferation of mammary epithelial cells, which has the potential to affect the tumorigenic process. In contrast, others studies have shown that inactivation of AhR inhibits cell proliferation. AhR-defective mutant cells (Hepa c1c12) derived from Hepa1c1c7 mouse hepatoma cells exhibit a prolonged doubling time and a higher percentage of cells in the G0/G1 phase compared to wild-type cells[27]. However, when AhR cDNA was introduced, the doubling time of Hepa1c1c12 cells was restored similar to that of wild-type cells. Consistent with these results, silencing of AhR in HepG2 and MCF-7 cells resulted in cell arrest and an increase in the percentage of cells in the G0/G1 phase[28]. On the other hand, primary hepatocytes and embryonic fibroblasts obtained from an AhR-null mouse exhibit lower cell proliferation rates and increased levels of apoptosis compared to wild-type cells[29]. In both cases, higher levels of transforming growth factor (TGF) were detected in conditioned medium obtained from AhR-null cell cultures[30]. It was subsequently hypothesized that higher levels of the proliferation inhibitor TGF are due to a decrease in retinoic acid metabolism[6,31].
While it has been demonstrated that AhR acts as a positive regulator of cell proliferation, deregulation of apoptosis may also be important. This was showed in an initiation-promotion rat model. TCDD treatment was found to enhance hepatocellular proliferation and to reduce levels of apoptosis[32]. In vitro, TCDD inhibits the death of HepG2 cells by an etoposide-induced mechanism[33]. Similar results were reported when MCF10A and Huh-7 cell cultures were irradiated with UVC light[34,35]. Interestingly, in both treatment-induced apoptosis models, lower levels of the well-studied tumor suppressor, p53, were detected. P53 is a protein that promotes DNA repair and/or apoptosis, and the loss of its function has observed in several human cancers. Pääjärvi et al[36] have proposed a mechanism by which TCDD and PAHs decrease levels of p53, in which TCDD activates the ubiquitin ligase, Mdm2, which in turn degrades p53. More recently, we have shown that activation of AhR increases levels of Ube2l3, an ubiquitin-conjugating enzyme, thereby enhancing ubiquitination and degradation of p53 and attenuating apoptosis[11].
In contrast with the above reports, AhR has also been identified as a negative regulator of cell proliferation. For example, treatment of 5L hepatoma cells with TCDD leads to the arrest of cells in the S phase[37]. However, when AhR deficient variants were used, an inhibition of cell growth by TCDD was not observed, suggesting that this effect is mediated by AhR[38]. Similar results were reported when MCF-7 cells were treated with TCDD[39]. More recently, Laiosa et al[40] observed an increase in the numbers of thymocytes in the G1 phase following TCDD treatment. Negative regulation of cell proliferation by AhR has also been observed in the absence of exogenous ligands. Overexpression of AhR was found to inhibit the growth of Jurkat T cells by arresting cells in the G1 phase and increasing levels of apoptosis[41].
Regarding the anti-proliferative activity of AhR, it appears to be mediated by its function as a transcription factor and by its capacity to interact with other proteins. In particularly, in 5L cells, activation of AhR induces the transcription and translation of the cell cycle inhibitor p27Kip1[42]. A similar result was obtained in human neuronal cells, where TCDD treatment induced p27Kip1 expression and led to an inhibition of cell proliferation and hypophosphorylation of retinoblastoma (pRb) protein[43]. Alternatively, when AhR directly interacts with other proteins, it can modulate several cell processes as well. Puga et al[44] showed that, when AhR interacts with the tumor suppressor protein, pRb, the ability of pRb to inhibit the transcriptional activity of E2F is enhanced, thereby leading to an arrest of the cell cycle in the G1 phase.
When investigating the mechanisms that mediate cancer development, it can be difficult to separate genetic factors from immunological factors, particularly regarding the involvement of AhR which has the ability to regulate both events independently. As previously discussed, AhR has the capacity to regulate the cell cycle and induce apoptosis, while also affecting both the induction and repression of carcinogenesis. During the carcinogenic process, the response of the immune system towards tumor cells is an important component, in addition to the role of the transformed cells themselves. The immune response can also vary from individual to individual. AhR has been shown to regulate many aspects of immune cell responses to tumor development[45,46], and a list of relevant studies are provided in Table 1.
| Cell type | AhR-dependent effect | Ref. |
| Th | Absence of AhR increases secretion of inflammatory cytokines (IFN-γ, IL-12) | [71] |
| Treg | Activation of AhR by TCDD increases their proliferation | [58] |
| Tr1 | IL-27-induces AhR activity, which in combinatio with c-Maf, suppresses their differentiation | [72] |
| B cells | TCDD enhances IgM secretion and inhibits the differentiation of plasma cells | [73] |
| DCs | TCDD reduces the number of splenic DCs | [74] |
| Macrophage | AhR-STAT1 interactions suppress the activation and differentiation of macrophage, and induces the IgA receptor FcαRI. TCDD reduces CD11a expression. | [49,75] [76] |
Many authors consider that AhR regulates the immune system mainly by balancing levels of Th17 and Treg cells. For example, AhR signalling influences the differentiation of Treg cells by activating the TGF- signalling pathway[47]. In this case, the presence of TGF-induces Treg cells, and in combination with the presence of IL-6 in the microenvironment, differentiation of Th17 cells is favored[48]. Another pathway involves the interaction of AhR with the signal transduction activator transcriptional factor (STAT)-1, which can modify the activation and differentiation of macrophage depending on the microenvironment of the responsive cells[49]. Consequently, differentiation of Th17/Treg cells is affected[50]. Another aspect of the immune response that is affected by AhR involves interaction between AhR and RelB, a transcription factor necessary for the differentiation and function of dendritic cells (DCs)[51]. AhR also participates in regulating NF-κB (reviewed in[52]), a ubiquitous transcription factor that regulates many chemical mediators of the immune system. Consequently, NF-κB can affect different outcomes when an immune response is elicited. There is also evidence to indicate that a regulatory loop of AhR can control cytokine production, and these cytokines can modulate the expression of some CYPs[53], thereby affecting the outcome of an immune response.
Despite the complex interactions that exist between the immune system and AhR, some researchers have also identified AhR as an immune regulator that modulates the development of cancer as a result of AhR-independent processes. The endogenous ligand, kynurenine (kyn), which is produced from tryptophan by cancer cells, can act on AhR to facilitate the evasion of brain tumors from immune surveillance[54]. In a different, yet related, strategy, AhR is required to induce the expression of indoleamine 2,3-dioxygenase (IDO), an immunosuppressive enzyme that metabolizes tryptophan into Kyn in DCs[55]. As a result of this interaction, an immunosuppressive microenvironment is created which facilitates tumor evasion.
The ability of AhR to modulate an immune response to tumor cells mostly depends on the type of ligand that engages AhR, as well as the physiological conditions of individuals[56,57]. The duration of AhR stimulation is also a factor, and can determine the outcome of the immune response generated. This has been observed with FICZ (6-formylindolo (3, 2-b) carbazole) and TCDD ligands[58], and also among endogenous ligands such as Lipoxin A4 and bilirubin, which can lead to opposite effects in the same disease model[59,60].
A dual role for AhR in the outcome of infection diseases has also been observed in several experimental models. Just the presence of AhR can control inflammation in parasitic diseases such as experimental Toxoplasmosis[61] and experimental Leishmaniasis[62], as well as in bacterial infections such as Citrobacterrodentium[63] and Streptococcus pneumonia[64]. In other models, activation of AhR has been shown to induce inflammation and a more rapid clearance of infection, as observed in an animal model infected with Listeria monocytogenes[65]. Regarding viruses, AhR also plays a key role in the outcome of the infection. Activation of AhR by TCDD was found to decrease the survival rate of animals already infected with influenza virus[66], it increased the viral burden of human cytomegalovirus[67], and it increased the replication rate of immunodeficiency virus type 1[68].
With AhR able to control the balance of inflammation and immune suppression by modulating levels of Th17/Th22 subpopulations, AhR also influences the outcome of certain degenerative disease models, particularly autoimmune-based diseases. Specific immune microenvironments are also dependent on levels of TGF and IL-6, thus, the balance of Th17/Treg cells can be affected (Table 2).
| Degenerative disease | AhR-dependent effect | Ref. |
| Experimental autoimmune encephalomyelitis | TCDD suppresses the disease by inducing T reg cells FICZ exacerbates the disease | [58] |
| GVHD | TCDD prevents the disease by generating T reg cells | [46] |
| Colitis | FICZ exacerbates disease | [77] |
| Collagen-induced arthritis | AhR exacerbates the diseas | [78] |
| Autoimmune uveoretinitis | TCDD suppresses the disease | [79] |
| Spontaneous autoimmune diabetes | TCDD suppresses the disease | [80] |
| Experimental multiple sclerosis | AhR activation reduces inflammation via Tr1 induction | [72] |
| Experimental lupus | Induction of Tr1 by AhR activation stops the inflammatory process | [81] |
| Rheumatoid arthritis | AhR activation by TCDD exacerbates the disease | [82] |
Several studies have established that AhR mediates functions in addition to xenobiotic detoxification. In this review, the ability of AhR to regulate cell proliferation and homeostasis of the immune system were described, thereby supporting the link between AhR and cancer and inflammation. In addition, the molecular mechanisms associated with, or predicted for, the actions of AhR suggest that this receptor is a potential target for the treatment of cancer and immune disorders. However, further research is needed to more completely characterize this protein. In particular, it remains unclear what determines whether AhR will behave as an oncogene or a tumor suppressor, or as a pro-inflammatory or anti-inflammatory factor. Furthermore, endogenous ligands of AhR remain to be identified, and this will facilitate the development of novel AhR ligands, agonists, and antagonists that do not have toxic side effects. Currently, there are several drugs (approved for use by the United States FDA) that present agonist or antagonist activity towards AhR. These include omeprazole and mexiletine, which may be useful as therapeutic drugs for pathologies where AhR has been shown to have a role. Omeprazole has been found to be a potent inhibitor of migrating breast cancer cells, and this effect is AhR-dependent[69].
Finally, it is worth noting that the transactivation domain sequence of human AhR (hAhR) and mouse AhR (mAhR) share 58% similarity, and hAhR exhibits a 10 fold lower affinity for TCDD compared with mAhR. However, the opposite result is observed for endogenous ligands such as indirubin[70], that have higher affinity to hAhR than to mAhR. These observations are relevant for the development of pharmacological drugs that target AhR, a receptor whose relevance to human disease continues to be elucidated.
P- Reviewer: Chan WK S- Editor: Song XX L- Editor: A E- Editor: Liu XM
| 1. | 1 Nebert DW, Gelboin HV. The in vivo and in vitro induction of aryl hydrocarbon hydroxylase in mammalian cells of different species, tissues, strains, and developmental and hormonal states. Arch Biochem Biophys. 1969;134:76-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 313] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Legraverend C, Hannah RR, Eisen HJ, Owens IS, Nebert DW, Hankinson O. Regulatory gene product of the Ah locus. Characterization of receptor mutants among mouse hepatoma clones. J Biol Chem. 1982;257:6402-6407. [PubMed] [Cited in This Article: ] |
| 3. | Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936-4946. [PubMed] [Cited in This Article: ] |
| 4. | Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141:131-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Gonzalez FJ, Fernandez-Salguero P. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metab Dispos. 1998;26:1194-1198. [PubMed] [Cited in This Article: ] |
| 6. | Shoda J, Miura T, Utsunomiya H, Oda K, Yamamoto M, Kano M, Ikegami T, Tanaka N, Akita H, Ito K. Genipin enhances Mrp2 (Abcc2)-mediated bile formation and organic anion transport in rat liver. Hepatology. 2004;39:167-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shimba S, Watabe Y. Crosstalk between the AHR signaling pathway and circadian rhythm. Biochem Pharmacol. 2009;77:560-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O, Santo N, Tohkin M, Furukawa Y, Gonzalez FJ. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol Appl Pharmacol. 2008;229:10-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Kerkvliet NI. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem Pharmacol. 2009;77:746-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 407] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 11. | Reyes-Hernández OD, Mejía-García A, Sánchez-Ocampo EM, Cabañas-Cortés MA, Ramírez P, Chávez-González L, Gonzalez FJ, Elizondo G. Ube2l3 gene expression is modulated by activation of the aryl hydrocarbon receptor: implications for p53 ubiquitination. Biochem Pharmacol. 2010;80:932-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97:779-782. [PubMed] [Cited in This Article: ] |
| 13. | Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, Pesatori AC. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol. 2001;153:1031-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 373] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 14. | Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2011;119:1700-1705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Hooiveld M, Heederik DJ, Kogevinas M, Boffetta P, Needham LL, Patterson DG, Bueno-de-Mesquita HB. Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. Am J Epidemiol. 1998;147:891-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Koliopanos A, Kleeff J, Xiao Y, Safe S, Zimmermann A, Büchler MW, Friess H. Increased arylhydrocarbon receptor expression offers a potential therapeutic target for pancreatic cancer. Oncogene. 2002;21:6059-6070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Ma JX, Zhang KL, Liu X, Ma YL, Pei LN, Zhu YF, Zhou L, Chen XY, Kong QY, Li H. Concurrent expression of aryl hydrocarbon receptor and CYP1A1 but not CYP1A1 MspI polymorphism is correlated with gastric cancers raised in Dalian, China. Cancer Lett. 2006;240:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kashani M, Steiner G, Haitel A, Schaufler K, Thalhammer T, Amann G, Kramer G, Marberger M, Schöller A. Expression of the aryl hydrocarbon receptor (AhR) and the aryl hydrocarbon receptor nuclear translocator (ARNT) in fetal, benign hyperplastic, and malignant prostate. Prostate. 1998;37:98-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, Hanberg A, Poellinger L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA. 2002;99:9990-9995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L, Schwarz M. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64:4707-4710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Shimba S, Komiyama K, Moro I, Tezuka M. Overexpression of the aryl hydrocarbon receptor (AhR) accelerates the cell proliferation of A549 cells. J Biochem. 2002;132:795-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Singh SS, Hord NG, Perdew GH. Characterization of the activated form of the aryl hydrocarbon receptor in the nucleus of HeLa cells in the absence of exogenous ligand. Arch Biochem Biophys. 1996;329:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Ikuta T, Kobayashi Y, Kawajiri K. Cell density regulates intracellular localization of aryl hydrocarbon receptor. J Biol Chem. 2004;279:19209-19216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Tannheimer SL, Barton SL, Ethier SP, Burchiel SW. Carcinogenic polycyclic aromatic hydrocarbons increase intracellular Ca2+ and cell proliferation in primary human mammary epithelial cells. Carcinogenesis. 1997;18:1177-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Xie G, Peng Z, Raufman JP. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1006-G1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-KB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498-5506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Ma Q, Whitlock JP. The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol. 1996;16:2144-2150. [PubMed] [Cited in This Article: ] |
| 28. | Abdelrahim M, Smith R, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol Pharmacol. 2003;63:1373-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Zaher H, Fernandez-Salguero PM, Letterio J, Sheikh MS, Fornace AJ, Roberts AB, Gonzalez FJ. The involvement of aryl hydrocarbon receptor in the activation of transforming growth factor-beta and apoptosis. Mol Pharmacol. 1998;54:313-321. [PubMed] [Cited in This Article: ] |
| 30. | Elizondo G, Fernandez-Salguero P, Sheikh MS, Kim GY, Fornace AJ, Lee KS, Gonzalez FJ. Altered cell cycle control at the G (2)/M phases in aryl hydrocarbon receptor-null embryo fibroblast. Mol Pharmacol. 2000;57:1056-1063. [PubMed] [Cited in This Article: ] |
| 31. | Andreola F, Hayhurst GP, Luo G, Ferguson SS, Gonzalez FJ, Goldstein JA, De Luca LM. Mouse liver CYP2C39 is a novel retinoic acid 4-hydroxylase. Its down-regulation offers a molecular basis for liver retinoid accumulation and fibrosis in aryl hydrocarbon receptor-null mice. J Biol Chem. 2004;279:3434-3438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Stinchcombe S, Buchmann A, Bock KW, Schwarz M. Inhibition of apoptosis during 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated tumour promotion in rat liver. Carcinogenesis. 1995;16:1271-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Ambolet-Camoit A, Bui LC, Pierre S, Chevallier A, Marchand A, Coumoul X, Garlatti M, Andreau K, Barouki R, Aggerbeck M. 2,3,7,8-tetrachlorodibenzo-p-dioxin counteracts the p53 response to a genotoxicant by upregulating expression of the metastasis marker agr2 in the hepatocarcinoma cell line HepG2. Toxicol Sci. 2010;115:501-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Park S, Matsumura F. Characterization of anti-apoptotic action of TCDD as a defensive cellular stress response reaction against the cell damaging action of ultra-violet irradiation in an immortalized normal human mammary epithelial cell line, MCF10A. Toxicology. 2006;217:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Chopra M, Dharmarajan AM, Meiss G, Schrenk D. Inhibition of UV-C light-induced apoptosis in liver cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2009;111:49-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Pääjärvi G, Viluksela M, Pohjanvirta R, Stenius U, Högberg J. TCDD activates Mdm2 and attenuates the p53 response to DNA damaging agents. Carcinogenesis. 2005;26:201-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Wiebel FJ, Klose U, Kiefer F. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in vitro: H4IIEC3-derived 5L hepatoma cells as a model system. Toxicol Lett. 1991;55:161-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Göttlicher M, Cikryt P, Wiebel FJ. Inhibition of growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin in 5L rat hepatoma cells is associated with the presence of Ah receptor. Carcinogenesis. 1990;11:2205-2210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Vogel C, Abel J. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on growth factor expression in the human breast cancer cell line MCF-7. Arch Toxicol. 1995;69:259-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, Silverstone AE. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol. 2003;171:4582-4591. [PubMed] [Cited in This Article: ] |
| 41. | Ito T, Tsukumo S, Suzuki N, Motohashi H, Yamamoto M, Fujii-Kuriyama Y, Mimura J, Lin TM, Peterson RE, Tohyama C. A constitutively active arylhydrocarbon receptor induces growth inhibition of jurkat T cells through changes in the expression of genes related to apoptosis and cell cycle arrest. J Biol Chem. 2004;279:25204-25210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Kolluri SK, Weiss C, Koff A, Göttlicher M. p27 (Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999;13:1742-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 273] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 43. | Jin DQ, Jung JW, Lee YS, Kim JA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits cell proliferation through arylhydrocarbon receptor-mediated G1 arrest in SK-N-SH human neuronal cells. Neurosci Lett. 2004;363:69-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Puga A, Barnes SJ, Dalton TP, Chang Cy, Knudsen ES, Maier MA. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J Biol Chem. 2000;275:2943-2950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol. 2013;25:335-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 46. | Quintana FJ. The aryl hydrocarbon receptor: a molecular pathway for the environmental control of the immune response. Immunology. 2013;138:183-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382-2391. [PubMed] [Cited in This Article: ] |
| 48. | Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 997] [Cited by in F6Publishing: 956] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 49. | Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027-2035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 50. | Lee JS, Cella M, Colonna M. AHR and the Transcriptional Regulation of Type-17/22 ILC. Front Immunol. 2012;3:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Lee JA, Hwang JA, Sung HN, Jeon CH, Gill BC, Youn HJ, Park JH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates functional differentiation of mouse bone marrow-derived dendritic cells Downregulation of RelB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2007;173:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Tian Y. Ah receptor and NF-KB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 53. | Liptrott NJ, Penny M, Bray PG, Sathish J, Khoo SH, Back DJ, Owen A. The impact of cytokines on the expression of drug transporters, cytochrome P450 enzymes and chemokine receptors in human PBMC. Br J Pharmacol. 2009;156:497-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1207] [Cited by in F6Publishing: 1320] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 55. | Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961-19966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 507] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 56. | Anderson G, Beischlag TV, Vinciguerra M, Mazzoccoli G. The circadian clock circuitry and the AHR signaling pathway in physiology and pathology. Biochem Pharmacol. 2013;85:1405-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Martinez JM, Afshari CA, Bushel PR, Masuda A, Takahashi T, Walker NJ. Differential toxicogenomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin in malignant and nonmalignant human airway epithelial cells. Toxicol Sci. 2002;69:409-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1263] [Cited by in F6Publishing: 1339] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 59. | Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594-7600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Sinal CJ, Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol. 1997;52:590-599. [PubMed] [Cited in This Article: ] |
| 61. | Sanchez Y, Rosado Jde D, Vega L, Elizondo G, Estrada-Muñiz E, Saavedra R, Juárez I, Rodríguez-Sosa M. The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis. J Biomed Biotechnol. 2010;2010:505694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Elizondo G, Rodríguez-Sosa M, Estrada-Muñiz E, Gonzalez FJ, Vega L. Deletion of the aryl hydrocarbon receptor enhances the inflammatory response to Leishmania major infection. Int J Biol Sci. 2011;7:1220-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 64. | Wang T, Wyrick KL, Pecka MR, Wills TB, Vorderstrasse BA. Mechanistic exploration of AhR-mediated host protection against Streptococcus pneumoniae infection. Int Immunopharmacol. 2012;13:490-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Shi LZ, Faith NG, Nakayama Y, Suresh M, Steinberg H, Czuprynski CJ. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J Immunol. 2007;179:6952-6962. [PubMed] [Cited in This Article: ] |
| 66. | Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. J Immunol. 2007;179:247-255. [PubMed] [Cited in This Article: ] |
| 67. | Murayama T, Inoue M, Nomura T, Mori S, Eizuru Y. 2,3,7,8-Tetrachlorodibenzo-p-dioxin is a possible activator of human cytomegalovirus replication in a human fibroblast cell line. Biochem Biophys Res Commun. 2002;296:651-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Ohata H, Tetsuka T, Hayashi H, Onozaki K, Okamoto T. 3-methylcholanthrene activates human immunodeficiency virus type 1 replication via aryl hydrocarbon receptor. Microbiol Immunol. 2003;47:363-370. [PubMed] [Cited in This Article: ] |
| 69. | Jin UH, Lee SO, Safe S. Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J Pharmacol Exp Ther. 2012;343:333-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Flaveny CA, Murray IA, Perdew GH. Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicol Sci. 2010;114:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 71. | Rodríguez-Sosa M, Elizondo G, López-Durán RM, Rivera I, Gonzalez FJ, Vega L. Over-production of IFN-gamma and IL-12 in AhR-null mice. FEBS Lett. 2005;579:6403-6410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 566] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 73. | Lu H, Crawford RB, Suarez-Martinez JE, Kaplan BL, Kaminski NE. Induction of the aryl hydrocarbon receptor-responsive genes and modulation of the immunoglobulin M response by 2,3,7,8-tetrachlorodibenzo-p-dioxin in primary human B cells. Toxicol Sci. 2010;118:86-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Vorderstrasse BA, Kerkvliet NI. 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects the number and function of murine splenic dendritic cells and their expression of accessory molecules. Toxicol Appl Pharmacol. 2001;171:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Funatake CJ, Dearstyne EA, Steppan LB, Shepherd DM, Spanjaard ES, Marshak-Rothstein A, Kerkvliet NI. Early consequences of 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on the activation and survival of antigen-specific T cells. Toxicol Sci. 2004;82:129-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Pinel-Marie ML, Louarn L, Desmots S, Fardel O, Sparfel L. Aryl hydrocarbon receptor-dependent induction of the IgA receptor FcαRI by the environmental contaminant benzo(a)pyrene in human macrophages. Toxicology. 2011;290:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237-248, 248.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 78. | Nakahama T, Kimura A, Nguyen NT, Chinen I, Hanieh H, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc Natl Acad Sci USA. 2011;108:14222-14227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Zhang L, Ma J, Takeuchi M, Usui Y, Hattori T, Okunuki Y, Yamakawa N, Kezuka T, Kuroda M, Goto H. Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Invest Ophthalmol Vis Sci. 2010;51:2109-2117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Kerkvliet NI, Steppan LB, Vorachek W, Oda S, Farrer D, Wong CP, Pham D, Mourich DV. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1:539-547. [PubMed] [Cited in This Article: ] |
| 81. | Wu HY, Quintana FJ, da Cunha AP, Dake BT, Koeglsperger T, Starossom SC, Weiner HL. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One. 2011;6:e23618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Kobayashi S, Okamoto H, Iwamoto T, Toyama Y, Tomatsu T, Yamanaka H, Momohara S. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:1317-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |