Published online Jun 16, 2023. doi: 10.5497/wjp.v12.i3.18
Peer-review started: January 20, 2023
First decision: April 28, 2023
Revised: May 10, 2023
Accepted: May 31, 2023
Article in press: May 31, 2023
Published online: June 16, 2023
The therapeutic potential of diet, dietary supplements, herbal remedies, and nutraceuticals for treatment of depression and anxiety is being increasingly explored. In this commentary, we discuss the recent findings on the antidepressant potential of silymarin (SILY) in mice and present an alternative approach. We highlight the extensive research on another phytochemical, curcumin, for the treatment of depression and anxiety. Finally, we suggest a future application, which investigates the potential synergistic effects of combined treatment with SILY and curcumin for depression.
Core Tip: There are several reviews focused on the role of silymarin (SILY) in chronic diseases, however, there is a paucity of literature reviewing the potential antidepressant effects of SILY. This commentary serves as a discussion of the recent findings regarding the antioxidant, anti-inflammatory, and antidepressant-like potential of SILY in mice and as a catalyst for future discovery in phytochemistry.
- Citation: Manhard CE, Lucke-Wold B. Commentary: Discussing the antidepressant potential of silymarin. World J Pharmacol 2023; 12(3): 18-24
- URL: https://www.wjgnet.com/2220-3192/full/v12/i3/18.htm
- DOI: https://dx.doi.org/10.5497/wjp.v12.i3.18
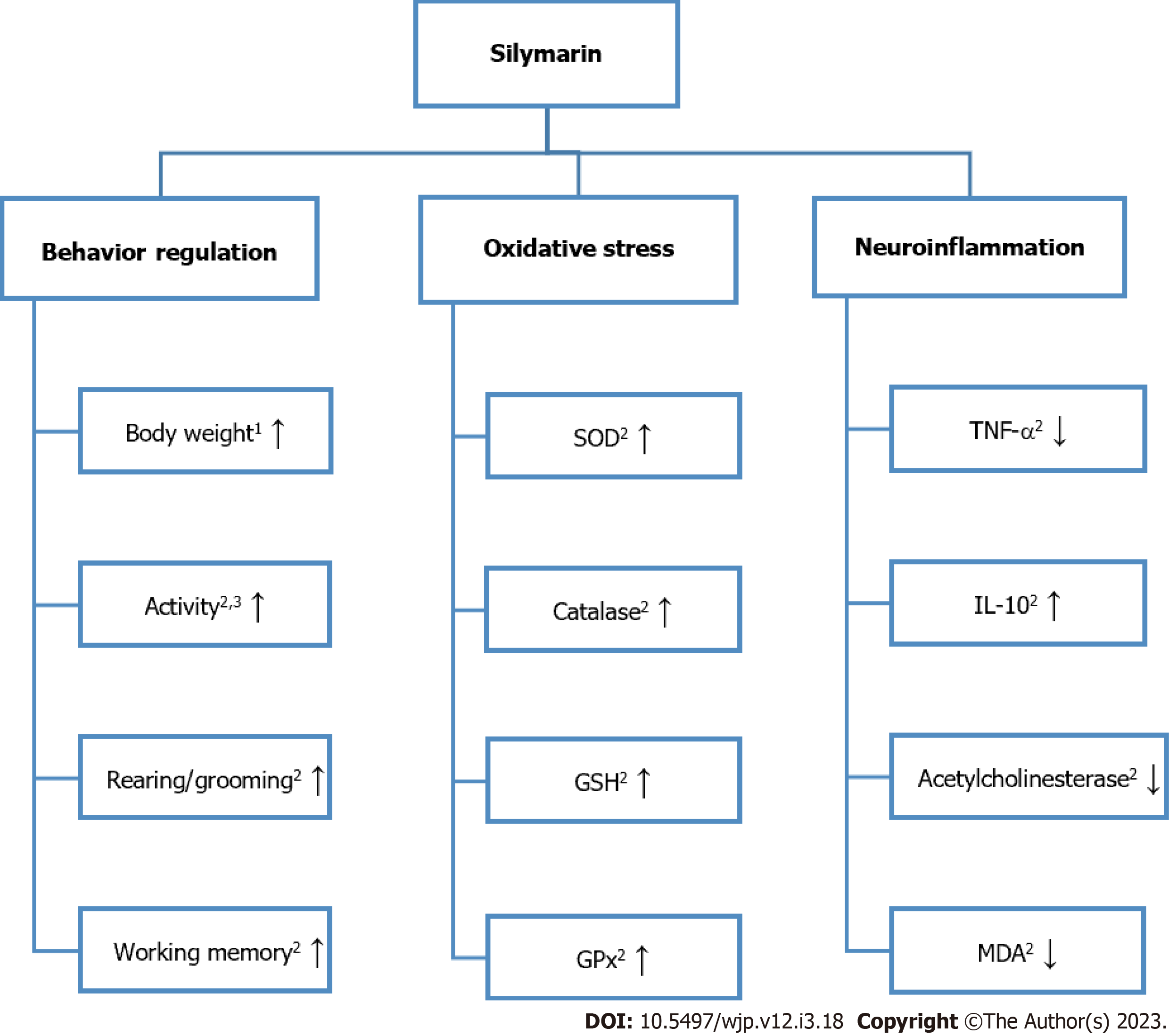
The paper titled “Antidepressant-like potential of silymarin and silymarin -sertraline combination in mice: Highlighting effects on behavior, oxidative stress, and neuroinflammation” by Onaolapo et al[1] demonstrates the ability of silymarin (SILY) alone or in conjunction with oral sertraline to regulate behavior, oxidative stress, and neuroinflammation in dexamethasone (DEX)-induced depression in mice[2]. A summarization of this study’s main findings regarding the effects of SILY alone can be found in Figure 1.
SILY, which is an active compound from the Silybum marianum L. Gaernt. herb, commonly called milk thistle, has antifibrotic, anti-inflammatory, and antioxidant properties[2-4]. Due to SILYs ability to prevent free radical formation and modify enzymes, its hepatoprotective effects have been well studied[5,6]. More recently, research has explored SILYs potential as a treatment for alcoholic liver cirrhosis[7]. Emerging evidence has suggested SILY’s anti-viral[8], anti-cancer[9], anti-Alzheimer’s disease[10], anti-Parkinson’s disease[11], and anti-diabetic[12] therapeutic effects.
Depression, a complex neuropsychiatric disorder, is a leading cause of disability, a large contributor to global disease burden, and is associated with high suicidality[13]. One longstanding hypothesis for the development of depression is the “serotonin hypothesis”[14]. This hypothesis posits that serotonin (5-hydroxytyptamine) plays a critical role in the pathophysiology of depression[15]. Conventional treatment of depression with serotonin reuptake inhibitors (SSRIs) acts by blocking the reuptake of serotonin on the presynaptic neuron, thus increasing the amount of serotonin in the synaptic cleft and postsynaptic neuronal activity[16].
As the precise etiology of depression remains unknown, researchers have begun to investigate the potential benefits of mechanisms of action that go beyond neurotransmitter modulation such as the use of dietary supplements and herbal remedies[17,18]. Further, there is increasing evidence of the role of inflammation and oxidative stress in the pathogenesis of depression[19]. Several studies have demon-strated activated inflammatory pathways in patients with depression with increased acetylcholinesterase activity, malondialdehyde (MDA), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-2, and IL-6, and decreased IL-10[20-26]. Interestingly, these inflammatory markers may also directly impact the release of serotonin and/or the production of serotonin[27,28]. Radical-induced oxidative stress has also been shown to be linked to depression, with increased superoxide dismutase (SOD) and decreased glutathione (GSH) peroxidase (GPx), GSH, and catalase in both nonclinical and clinical studies[20,29,30].
Given the association of inflammation and oxidative stress with depression, Onaolapo et al[1] sought to elucidate the potential antidepressant effect of SILY extract alone or in conjunction with a well-known SSRI, sertraline in an animal model. DEX, which was used as the mice model of depression, has been demonstrated to be effective at creating a low levels of corticosteroids in the brain and inducing depression and anxiety-like behaviors[31,32]. A total of 10 treatment groups were included: 1 vehicle control group, 1 DEX group, 1 oral sertraline group, 1 SILY 140 mg/kg group and 1 SILY 280 mg/kg group, 1 DEX and sertraline group, 1 DEX and SILY 140 mg/kg group, 1 DEX and SILY 280 mg/kg group, 1 DEX, sertraline, and SILY 140 mg/kg group, and 1 DEX, sertraline, and SILY 280 mg/kg group.
Onaolapo et al[1] found that SILY administered alone with DEX or with sertraline and DEX reversed the DEX-induced changes in mouse body weight, open field locomotor activity, rearing, and grooming. Body weight increased when mice were given SILY alone as compared to the control or sertraline. While SILY affected body weight, there was no significant change in food intake with SILY alone when compared to control, sertraline, or DEX. Interestingly, Guo et al[10] found that SILY administration in mice that were fed a high fat diet resulted in a decrease in body weight and a clinical trial by Momeni et al[33] revealed that treatment with SILY following cisplatin was able to significantly increase weight back to baseline. Onaolapo et al[1] findings in conjunction with results from Guo et al[10] and Momeni et al[33] suggest that SILY modulates body weight in a way that returns body weight back to a normal value.
Additionally, there was also a SILY concentration dependent increase in locomotor activity. Rearing activity in the SILY group was not significantly different from control; however, low dose SILY rearing activity was significantly greater than sertraline. With regards to self-grooming behavior, only the low dose of SILY resulted in a significant increase in self-grooming behaviors in comparison to control and sertraline. While there was a relatively unclear dose relationship between SILY alone and grooming behaviors, it is important to note that there was a significant attenuation of DEX-induced decreases when mice were administered SILY at both doses with DEX or SILY at both doses with sertraline and DEX. This corroborates Ashraf et al[34] findings that SILY administration at 200 mg/kg in chronically stressed mice resulted in a significant increase in grooming time.
Onaolapo et al[1] reported that spatial working memory, tested using the radial arm maze and Y maze, was increased in mice administered SILY. SILY administered alone at both concentrations increased working memory scores as compared to the vehicle control and sertraline, and SILY administered with sertraline and DEX or alone with DEX attenuated the DEX-induced reduction in working memory. They also found that SILY was able to positively affect anxiety-related behaviors tested using the elevated plus maze. The results demonstrated that SILY alone increased the amount of time mice spent in the open arm and decreased the amount of time spent in the closed arm. SILY alone with DEX or with sertraline and DEX also counteracted the DEX-induced changes in time. El-Elimat et al[35] similarly demonstrated that SILY administration can prevent stress-induced memory impairments and improve anxiety-related behaviors in a rat model of post-traumatic stress disorder.
Results from the tail suspension and forced swim tests showed that SILY alone reduced the amount of immobility time significantly when compared to the vehicle control and sertraline groups. Further, SILY by itself or with sertraline attenuated the DEX-induced increases in immobility time in both tests. Lee et al[36] also assessed immobility time in forced swim tests in a rat model of stress and found a significant decrease in immobility time with administration of the main active ingredient in SILY.
Onaolapo et al[1] found mixed results for SILY’s effect on serum antioxidant status. Serum SOD levels increased significantly with both SILY concentrations and with DEX and high dose SILY. A decrease in serum SOD levels was found for DEX, DEX with sertraline, and both concentrations of SILY administered with DEX and sertraline. These results are interesting given that multiple studies have found increased SOD in patients with major depressive disorder[29,37-39]. With regards to catalase, serum concentrations increased significantly with both concentrations of SILY and DEX administered with SILY 280 mg/kg when compared to the vehicle control. Surprisingly, DEX with sertraline and DEX with SILY 140 mg/kg and sertraline resulted in a significant decrease in catalase serum concentrations when compared to the vehicle control. GSH also increased significantly with both concentrations of SILY and with DEX and SILY 280 mg/kg and DEX with SILY 280 mg/kg and sertraline when comparing to the vehicle control. The final antioxidant status measure, GPx, was found to have increased significantly with both concentrations of SILY and decrease with DEX and DEX with sertraline compared to the vehicle control. These mixed results put SILY’s effect on antioxidant status into question. However, another study by Ashraf et al[34] investigated SILY’s antioxidant potential in a chronically induced stress model of depression and found catalase, GSH, and SOD levels from both the hippocampus and cerebral cortex to be significantly greater in mice treated with SILY 200 mg/kg as compared to the control.
Inflammatory marker levels from the hippocampus and cerebral cortex were improved following SILY administration. TNF-α levels were lowered in mice administered SILY alone. Further, results showed SILY’s ability to mitigate the severely lowered TNF-α levels observed in DEX mice. IL-10 levels were increased in mice administered SILY alone. Results also demonstrated SILY’s ability to mitigate the decrease in IL-10 levels observed in DEX mice. Acetylcholinesterase activity was found to be significantly increased in DEX mice; however, this increase was again mitigated by SILY administration alone or in conjunction with sertraline. SILY at both concentrations also significantly reduced acetylcholinesterase activity as compared to the control and sertraline. Both concentrations of SILY were also found to significantly reduce the levels of MDA as compared to the control. SILY 280 mg/kg was also found to significantly reduce the levels of MDA when compared to sertraline, as well. As with the other inflammatory markers, SILY alone or with sertraline attenuated the increase in MDA levels observed with DEX administration. The levels of the brain antioxidant enzymes tested, GSH and GPx, were both increased with both concentrations of SILY when compared to control and to sertraline. Further, SILY alone or with sertraline also mitigated the decrease in GSH and GPx induced by DEX. Ashraf et al[34] similarly found improved levels of inflammatory markers in stressed mice, with significantly lowered IL-1β and TNF-α compared to the control. Thakare et al[40] also found significantly lowered IL-6, MDA, and TNF-α in stressed mice when compared to the control. Onaolapo et al[1] finally examined the morphology of the cerebral cortex and hippocampus. Cerebral cortex and hippocampal histomorphology demonstrated DEX-induced neuronal injury; however, when mice were administered DEX, sertraline, and SILY the histology appeared to have normalized suggesting SILY and sertraline’s potential neuronal protective effects.
Overall, Onaolapo et al[1] conducted a well-designed study, which elucidated the effects of SILY on depressive and anxiety related behaviors, neuroinflammation, oxidative stress, and neuronal injury. The number of groups, randomization schemes, and the inclusion of 10 animals per group indicates strong experimental design. Results regarding its effects on oxidative stress were unclear, however, existing literature does suggest SILY’s ability to modulate oxidative stress[34,40]. As the authors pointed out, the lack of assessment of glucocorticoid levels limits the study’s ability to determine SILY’s impact on the hypothalamus-pituitary-adrenocortical axis, which is implicated in depression and anxiety[41]. Further, the authors did not study SILY’s effects on monoamine levels in the brain and failed to comment on the sex-dependent effects of SILY, which have been demonstrated in a couple of studies[42,43]. Lastly, as this is an animal study, the results cannot reliably predict SILY’s effects in humans.
Another widely studied phytochemical, curcumin, has both nonclinical and clinical evidence suggesting its antidepressant potential[44]. Curcumin is main component of the spice turmeric or Curcuma longa, and like SILY, has been shown to have anti-inflammatory, antioxidant, and neuroprotective activities[45,46]. The literature has demonstrated curcumin’s ability to increase levels of monoamines in the central nervous system (CNS), inhibit glutamate release, decrease inflammation, improve oxidative stress, and attenuate symptoms associated with depression and anxiety[44].
A study by Bhutani et al[47] found that curcumin administration significantly reversed the increased depressive behaviors, low CNS monoamine levels, and increased monoamine oxidase activity observed in rats subjected to chronic unpredictable stress. Lin et al[48] showed curcumin’s ability to inhibit glutamate release in the prefrontal cortex of rats through the suppression of presynaptic voltage-gated calcium channels. Given that glutamate has been demonstrated to be elevated in patients with depression, these results suggest that curcumin inhibited glutamate release may provide antidepressant effects. Curcumin has also been shown to decrease inflammatory markers in a rat model of depression. Fan et al[49] found that pretreatment with curcumin repressed inflammatory processes including microglia activation and overexpression of IL-1β, IL-6, and TNF-α which were induced by chronic unpredictable stress. Further, in a 2015 clinical trial, Yu et al[50] found that chronic supplementation with curcumin decreased inflammatory cytokines, IL-1β and TNF-α, and salivary cortisol in depressed patients when compared to placebo. Naqvi et al[51] showed in an animal model that curcumin administration also improved oxidative stress, which is associated with depression[20]. This study found that curcumin administration in mice exposed to unpredictable chronic mild stress resulted in a reduction in depression and anxiety symptoms, lipid peroxidation, and antioxidant enzymatic activity. Similarly, Moradi Vastegani et al[52] demonstrated that curcumin pretreatment in mice administered lipopolysaccharide (LPS) resulted in significantly increased activity of SOD and GPx enzymes. Further, it attenuated the LPS-induced anxiety and depressive behavior.
An interesting application of the results from Onaolapo et al[1] could be a study on the potential synergistic effects of SILY and curcumin for depression. The combination of SILY and curcumin has been tested in both human colorectal cancer cell lines and in radiation induced kidney injury in rats[53,54]. One study by Montgomery et al[53] found that curcumin sensitized SILY’s effects in human colorectal cancer cell lines. Another study found the combination of curcumin and SILY for treatment of radiation induced kidney injury in rats to be potentially more effective than curcumin or SILY alone[54]. Based on the anti-inflammatory and antioxidant properties of both SILY and curcumin alone for depression and anxiety, and the combined effects tested in other disease states, combination therapy with SILY and curcumin may be a promising treatment with greater antidepressant potential. Further, in patients for whom SSRIs are not effective or are not preferred, this alternative combination treatment may offer benefit.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu XQ, China S-Editor: Wang JJ L-Editor: A P-Editor: Ji MX
| 1. | Onaolapo AY, Sulaiman H, Olofinnade AT, Onaolapo OJ. Antidepressant-like potential of silymarin and silymarin-sertraline combination in mice: Highlighting effects on behaviour, oxidative stress, and neuroinflammation. World J Pharmacol. 2022;11:27-47. [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. "Silymarin", a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci. 2011;14:308-317. [PubMed] [Cited in This Article: ] |
| 3. | El-Lakkany NM, Hammam OA, El-Maadawy WH, Badawy AA, Ain-Shoka AA, Ebeid FA. Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis. Parasit Vectors. 2012;5:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15:9-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Mirzaei E, Sabetian G, Masjedi M, Heidari R, Mirjalili M, Dehghanian A, Vazin A. The effect of silymarin on liver enzymes and antioxidant status in trauma patients in the intensive care unit: a randomized double blinded placebo-controlled clinical trial. Clin Exp Hepatol. 2021;7:149-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, Esquivel-Soto J, Esquivel-Chirino C, García-Luna Y González-Rubio M, Gayosso-de-Lucio JA, Morales-González JA. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6:144-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 205] [Article Influence: 20.5] [Reference Citation Analysis (2)] |
| 7. | Gillessen A, Schmidt HH. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv Ther. 2020;37:1279-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 8. | Liu CH, Jassey A, Hsu HY, Lin LT. Antiviral Activities of Silymarin and Derivatives. Molecules. 2019;24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457-4498. [PubMed] [Cited in This Article: ] |
| 10. | Guo H, Cao H, Cui X, Zheng W, Wang S, Yu J, Chen Z. Silymarin's Inhibition and Treatment Effects for Alzheimer's Disease. Molecules. 2019;24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Ullah H, Khan H. Anti-Parkinson Potential of Silymarin: Mechanistic Insight and Therapeutic Standing. Front Pharmacol. 2018;9:422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Kazazis CE, Evangelopoulos AA, Kollas A, Vallianou NG. The therapeutic potential of milk thistle in diabetes. Rev Diabet Stud. 2014;11:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | World Health Organization. Depressive disorder (depression). [cited 10 December 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/depression. [Cited in This Article: ] |
| 14. | Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015;14:158-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Meltzer HY. Role of serotonin in depression. Ann N Y Acad Sci. 1990;600:486-99; discussion 499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 147] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Nutt DJ, Forshall S, Bell C, Rich A, Sandford J, Nash J, Argyropoulos S. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur Neuropsychopharmacol. 1999;9 Suppl 3:S81-S86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Firth J, Teasdale SB, Allott K, Siskind D, Marx W, Cotter J, Veronese N, Schuch F, Smith L, Solmi M, Carvalho AF, Vancampfort D, Berk M, Stubbs B, Sarris J. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry. 2019;18:308-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 18. | Yeung KS, Hernandez M, Mao JJ, Haviland I, Gubili J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phytother Res. 2018;32:865-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2013;31:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 20. | Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 21. | Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology. 24:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 133] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Farooq RK, Asghar K, Kanwal S, Zulqernain A. Role of inflammatory cytokines in depression: Focus on interleukin-1β. Biomed Rep. 2017;6:15-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1916] [Cited by in F6Publishing: 1972] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 24. | Islam MR, Islam MR, Ahmed I, Moktadir AA, Nahar Z, Islam MS, Shahid SFB, Islam SN, Hasnat A. Elevated serum levels of malondialdehyde and cortisol are associated with major depressive disorder: A case-control study. SAGE Open Med. 2018;6:2050312118773953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 129] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Wang X, Li P, Ding Q, Wu C, Zhang W, Tang B. Observation of Acetylcholinesterase in Stress-Induced Depression Phenotypes by Two-Photon Fluorescence Imaging in the Mouse Brain. J Am Chem Soc. 2019;141:2061-2068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 27. | Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1037] [Cited by in F6Publishing: 1083] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 28. | Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8:365-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 31. | Karssen AM, Meijer OC, Berry A, Sanjuan Piñol R, de Kloet ER. Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology. 2005;146:5587-5595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Wu J, Li J, Gaurav C, Muhammad U, Chen Y, Li X, Chen J, Wang Z. CUMS and dexamethasone induce depression-like phenotypes in mice by differentially altering gut microbiota and triggering macroglia activation. Gen Psychiatr. 2021;34:e100529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Momeni A, Hajigholami A, Geshnizjani S, Kheiri S. Effect of silymarin in the prevention of Cisplatin nephrotoxicity, a clinical trial study. J Clin Diagn Res. 2015;9:OC11-OC13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Ashraf A, Mahmoud PA, Reda H, Mansour S, Helal MH, Michel HE, Nasr M. Silymarin and silymarin nanoparticles guard against chronic unpredictable mild stress induced depressive-like behavior in mice: involvement of neurogenesis and NLRP3 inflammasome. J Psychopharmacol. 2019;33:615-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | El-Elimat T, Alzoubi KH, AbuAlSamen MM, Al Subeh ZY, Graf TN, Oberlies NH. Silymarin Prevents Memory Impairments, Anxiety, and Depressive-Like Symptoms in a Rat Model of Post-Traumatic Stress Disorder. Planta Med. 2019;85:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Lee B, Choi GM, Sur B. Silibinin prevents depression-like behaviors in a single prolonged stress rat model: the possible role of serotonin. BMC Complement Med Ther. 2020;20:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Lukic I, Mitic M, Djordjevic J, Tatalovic N, Bozovic N, Soldatovic I, Mihaljevic M, Pavlovic Z, Radojcic MB, Maric NP, Adzic M. Lymphocyte levels of redox-sensitive transcription factors and antioxidative enzymes as indicators of pro-oxidative state in depressive patients. Neuropsychobiology. 2014;70:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Djordjević V. Superoxide Dismutase in Psychiatric Diseases. In: Reactive Oxygen Species. United Kingdom: IntechOpen, 2022. [Cited in This Article: ] |
| 39. | Russo AJ. Increased Serum Cu/Zn SOD in Individuals with Clinical Depression Normalizes After Zinc and Anti-oxidant Therapy. Nutr Metab Insights. 2010;3:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Thakare VN, Patil RR, Oswal RJ, Dhakane VD, Aswar MK, Patel BM. Therapeutic potential of silymarin in chronic unpredictable mild stress induced depressive-like behavior in mice. J Psychopharmacol. 2018;32:223-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 430] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 42. | Pereira-Figueiredo I, Sancho C, Carro J, Castellano O, López DE. The effects of sertraline administration from adolescence to adulthood on physiological and emotional development in prenatally stressed rats of both sexes. Front Behav Neurosci. 2014;8:260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Shokouhi G, Kosari-Nasab M, Salari AA. Silymarin sex-dependently improves cognitive functions and alters TNF-α, BDNF, and glutamate in the hippocampus of mice with mild traumatic brain injury. Life Sci. 2020;257:118049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 44. | Ramaholimihaso T, Bouazzaoui F, Kaladjian A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence-A Narrative Review. Front Psychiatry. 2020;11:572533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1048] [Cited by in F6Publishing: 1092] [Article Influence: 91.0] [Reference Citation Analysis (1)] |
| 46. | Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 711] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 47. | Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 48. | Lin TY, Lu CW, Wang CC, Wang YC, Wang SJ. Curcumin inhibits glutamate release in nerve terminals from rat prefrontal cortex: possible relevance to its antidepressant mechanism. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1785-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Fan C, Song Q, Wang P, Li Y, Yang M, Liu B, Yu SY. Curcumin Protects Against Chronic Stress-induced Dysregulation of Neuroplasticity and Depression-like Behaviors via Suppressing IL-1β Pathway in Rats. Neuroscience. 2018;392:92-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J Clin Psychopharmacol. 2015;35:406-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Naqvi F, Saleem S, Naqvi F, Batool Z, Sadir S, Tabassum S, Ahmed S, Liaquat L, Haider S. Curcumin lessens unpredictable chronic mild stress-induced depression and memory deficits by modulating oxidative stress and cholinergic activity. Pak J Pharm Sci. 2019;32:1893-1900. [PubMed] [Cited in This Article: ] |
| 52. | Moradi Vastegani S, Hajipour S, Sarkaki A, Basir Z, Parisa Navabi S, Farbood Y, Khoshnam SE. Curcumin mitigates lipopolysaccharide-induced anxiety/depression-like behaviors, blood-brain barrier dysfunction and brain edema by decreasing cerebral oxidative stress in male rats. Neurosci Lett. 2022;782:136697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Montgomery A, Adeyeni T, San K, Heuertz RM, Ezekiel UR. Curcumin Sensitizes Silymarin to Exert Synergistic Anticancer Activity in Colon Cancer Cells. J Cancer. 2016;7:1250-1257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Abdel-Magied N, Elkady AA. Possible curative role of curcumin and silymarin against nephrotoxicity induced by gamma-rays in rats. Exp Mol Pathol. 2019;111:104299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |